Abstract
Single phase perovskites, such as LaCoO3 and La1−xSrxCoO3, exhibit catalytic activity only in oxidation reactions. However, a mixture of La1−xSrxCoO3, SrCoOx and Co3O4 oxides presents both oxidation and reduction properties in the DeNOx reaction. To improve DeNOx activity, we have added 1–2 wt% of Ag to a mixture of nanosized oxides of La, Sr and Co. These mixtures (with and without Ag) were prepared by the sol–gel method using citric acid as the solvent. Two kinds of mixtures, A=La1−xSrxCoO3+Co3O4+SrCoOx and B=A+Ag, were characterized by x-ray diffraction (XRD) analysis, field emission scanning electron microscope (FESEM) and energy dispersive x-ray spectroscopy (EDS). Their catalytic activity was studied by temperature programmed surface reaction (TPSR). The results showed that (i) for catalyst A, the total C3H6-conversion temperature is in the range 210–500 °C, while for catalyst B that is 200–400 °C; (ii) the mixed oxide A exhibits a weak DeNOx activity, while the silver doping leads to a remarkable increase in the NOx-conversion from 29.4% (in the absence of Ag) to 82.4% at low temperature 220 °C in the lean burn condition.
Export citation and abstract BibTeX RIS

Content from this work may be used under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Catalysis plays a major role in reducing pollution and improving the atmosphere. Precious metals, such as Pt, Pd and Au, have high activity and stability for the oxidation of CO and volatile organic compounds (VOCs). However, owing to the scarcity and high cost of noble metals, worldwide efforts are being made to replace noble metal catalysts with other ones. To solve this problem, the catalytic properties of mixed metal oxides, typically oxides of Mn, Co and Fe, were studied. Among them, several perovskite-type ABO 3 (where position A is for rare earth metals and position B for transition metals) exhibit high activity in the deep oxidation of hydrocarbons and VOCs. A partial substitution of ions in position A and/or position B by A' and B' produces crystal lattice defects or creates oxygen vacancies in the lattice, and enhances the catalytic properties of the materials [1–4].
The data in [2, 3] show that several perovskite oxides exhibit activity in direct decomposition of NO x , but their activity is low and the effective temperature is high (above 700 °C). In an effort to reduce the temperature of NO x removal, many experiments have been carried out. The authors of [5–10] have indicated that the addition of silver to perovskite and/or oxides leads to an improvement in catalytic activity not only in oxidation (of CO, VOCs, aromatic hydrocarbons) and oxygen storage but also in the reactions of NO x removal at relatively low temperature. Miyadera [5] showed that several oxides ( Co /Al 2 O 3, Cu/Al 2 O 3, Ag/Al 2 O 3, V/Al 2 O 3 and Cr/Al 2 O 3) on an oxide alumina support exhibit different activities for the selective catalytic reduction (SCR) in humid conditions. The author found that 2% silver doped on alumina has the highest activity, with 80.5% NO x conversion at 400 °C in a reaction condition with 10% H 2 O.
The selective catalytic reduction of nitrogen oxides by hydrocarbons has attracted much attention because it has the potential ability to remove NO x from diesel exhaust and other oxygen-rich flue gases. In the preceding report [11], we found that the single phase perovskite La 1−x Sr x CoO 3 presents high catalytic activity only in the oxidation reaction. Meanwhile the mixed oxides, including perovskite phase and mono-/intermediate oxides, such as Co 3 O 4, La 2 O 3, SrO and SrCoO x , due to their multifunction, are able to catalyze the DeNO x reaction (removal of NO and NO 2).
In this work, in an effort to improve the catalytic properties of catalysts, the mixed oxides La 1−x Sr x CoO 3 and SrCoO x , La 2 O 3, SrO and Co 3 O 4 were doped with 2% weigh metal silver and were studied by a temperature programmed surface reaction (TPSR) in the lean burn condition.
2. Experimental
2.1. Catalyst preparation
Reagents such as La 2 O 3, Co(NO 3)2, SrCO 3, C 6 H 8 O 7·H 2 O, AgNO 3, NH 4 OH, CH 3 COOH and other chemicals were of analytical grade, the solutions being diluted with twice distillated water. Two kinds (A and B) of mixed oxides were prepared by the citrate sol–gel method from the following starting reagents, respectively: [La 2 O 3+Co(NO 3)2+SrCO 3] and [La 2 O 3+Co(NO 3)2+SrCO 3+AgNO 3].
The synthesis procedures were as follows. A weighed amount of La 2 O 3 and SrCO 3 were first dissolved in the presence of nitric and acetic acids, respectively, then mixed with a stoichiometric amount of Co(NO 3)2 and acidic citrate according to the following molar ratio: metal : citrate=3 : 2. The solution was adjusted to pH=5.5–6.5 with aqueous ammonia or acetic acid while stirring and heating to a temperature of 60–80 °C for 4 h. A dark-rose gel product was obtained. The gel was then heated to 100 °C for 24 h in air, calcined at 300 °C for 2 h, then the temperature was raised to 800 °C for 4 h. We produced sample A.
Sample B was similarly prepared from the reagent mixture mentioned above and 2% of AgNO 3.
2.2. Methods for catalyst characterization
X-ray diffraction analysis was carried out using a D5000 diffractometer with a Cu-Kα cathode (λ=1.5406 Å).
The FESEM images were obtained by Field Emission Scanning Electron Microscope using a Hitachi S4800 system.
The energy dispersive x-ray spectroscopy (EDS) analysis was performed using an FESEM Hitachi S4800 and a JEOL-JEM 2010 high-resolution transmission electron microscope, operating at 200 kV, with a PGT IMIX-PC EDS device.
The catalytic activity of the materials were studied by Temperature Programmed Surface Reaction (TPSR) with a special Siemens's instrument.
3. Results and discussion
3.1. Identification of materials by x-ray diffraction
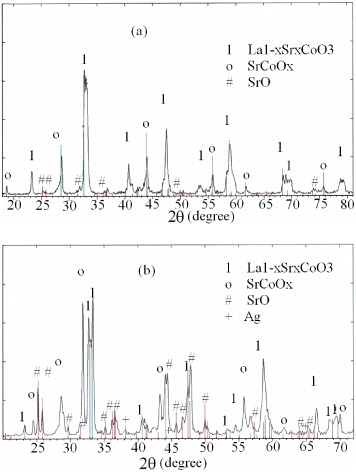
The XRD patterns of samples A and B are presented in figures 1(a) and 1(b), respectively. Figure 1(a) shows that sample A contained mainly the perovskite La 1−x Sr x CoO 3 phase mixed with a small amount of SrCoO x and SrO. In figure 1(b) for sample B, these are typical peaks of La 1−x−y Ag y Sr x CoO 3, SrCoO x , SrO and Ag. We can see that the intensity of the SrCoO x peak in figure 1(b) is higher than that in figure 1(a). This difference indicates that the quantity of SrCoO x in sample A is larger than that in sample B. This means that the presence of Ag leads to a change to the ratio of oxides contained in the sample. Moreover, ion Ag in sample B was converted into the metallic state. This phenomenon is similar to the result of Piotrowska and Landmesser [9], who considered the conversion of Ag 2 O into Ag when the calcining temperature was increased to more than 600 °C.
Figure 1 XRD patterns of sample A (a) and B (b).
3.2. Results of FESEM and EDS studies
Figure 2 presents the FESEM images of sample A (a) and B (b). It indicates that the particle size of both samples varied in the range 40–80 nm. Apart from the silver species, which are well dispersed into base metal oxides, we can still see numerous small metallic silver nanoparticles on the surface of mixed oxides. According to Shimizu et al [8], x-ray absorption near-edge structure (XANES) spectra showed that both Ag metal as the dominant silver species and Ag + ion as the minor silver species are present on the sample.
Figure 2 FESEM images of samples A (a) and B (b).
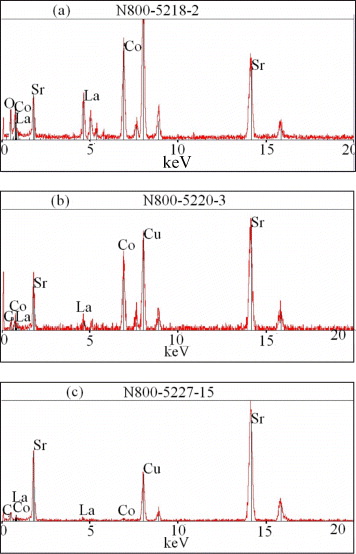
Through EDS studies on different points over both samples, we can observe various phases: the major phase of complex oxide with perovskite structure ABO 3/or AA' BO 3 and the transition mixed oxide SrCoO x and SrO. Figure 3 exhibits the existence of La 1−x Sr x CoO 3, SrCoO x and SrO in sample A. These EDS patterns are in agreement with the XRD results mentioned above.
Figure 3 EDS patterns of sample A (a) La 1−x Sr x CoO 3, (b) SrCoO x and (c) SrO.
Figure 4 shows that metallic silver is dispersed unevenly on the oxides La 1−x Sr x CoO 3, SrCoO x and SrO. Table 1 contains the data for three different points of sample B corresponding to EDS-patterns, figures 4(a)–(c), respectively: in figure 4(a), the Ag concentration is 2.82%, in figure 4(b) it is 0.95% and in figure 4(c) it is 0.84%. We can see the EDS result, which coincides with that of X-ray spectroscopy, as mentioned above: while the sample was calcined at 800 °C, the silver ion Ag + was converted into metallic silver Ag. The data in table 1 indicate the non-uniform chemical composition on the surface of the sample. The sample included not only perovskite La 1-x-y Ag y Sr x CoO 3, intermediate oxide SrCoO x , oxide SrO and metallic silver Ag but also oxides of La and Co, which had not been observed by x-ray spectroscopy.
Figure 4 EDS patterns of sample B (a) La 1−x Sr x CoO 3, (b) SrCoO x and (c) SrO.
Table 1. EDS data from the different points of sample B.
| O K | 38.42 | 75.09 | 22.59 | 61.45 | 22.70 | 60.59 | ||
| Co K | 25.54 | 13.55 | 14.14 | 10.44 | 23.94 | 17.35 | ||
| Sr L | 23.32 | 8.32 | 44.72 | 22.21 | 31.03 | 15.12 | ||
| Ag L | 2.82 | 0.82 | 0.95 | 0.38 | 0.84 | 0.33 | ||
| La L | 9.91 | 2.22 | 17.61 | 5.52 | 21.49 | 6.61 | ||
| Totals | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 |
3.3. Catalytic properties
Figures 5(a) and (b) present TPSR profiles of samples A and B=A+Ag, respectively. The TPSR were taken in the following conditions. The composition of the reactant mixture in the deNO x runs was 340 ppm NO x , 680 ppm C 3 H 6, 8 vol% oxygen, nitrogen as carrier gas. The total flow rate was 15 litres h −1, giving an hour space velocity of 75 000 h −1. In a typical run, 200 mg catalyst was introduced into the reactor. The catalyst was first pre-treated in air at 500 °C for 2 h. Then it was cooled to room temperature. The reaction was carried out with a heating rate of 10 K min −1 in a flowing reaction mixture.
Figure 5 TPSR profiles over catalyst A (a) and B (b).
Figure 5 shows that the behaviors of the C 3 H 6 oxidation and NO x removal for the two catalysts A and B are different. This can be explained by analyzing the TPSR data in tables 2 and 3, as follows.
Scheme 1 The three-cycle model for DeNO x reactions in lean burn conditions [12].
Table 2. Temperature and time of C 3 H 6 oxidation over catalysts A and B.
| A=La 1−x Sr x CoO 3+SrCoO x +SrO | 200–500 | 57 | 84 |
| B=A+Ag | 200–400 | 40 | 62 |
Table 3. Data of process removing NO x over catalyst A and B.
| Reaction starting time (min) | 45 | 63 | 74 | 25 | 40 | 52 |
| Temperature of maximum decomposition (°C) | 120 | 220 | 350 | 120 | 220 | 330 |
| Conversion NO x (%) | 23.5 | 29.4 | 41 | 76.5 | 82.4 | 50 |
3.3.1. C3 H6 oxidation process
Over sample A, the C 3 H 6 oxidation begins from 200 °C and total oxidation at 500 °C, while over B, the C 3 H 6 oxidation runs in the range 200–400 °C (table 2). Moreover, both the starting and finishing times of the reaction over B are earlier than those of A. Therefore, Ag doped in the catalyst does not change the starting reaction temperature but decreases the total oxidation temperature of propane on 100 °C and increases the reaction speed over B.
According to Luo et al and Shimizu et al [7, 8], doping silver in the composite oxide catalysts have some combination with the base metal, such as (Ag–Co), which may create a cooperative action, breaking the activation energy barriers of the individual catalyst. This results in a decrease in the activation energy of the rate-determining step and the lattice oxygen of the Ag-doped oxide catalysts is much more reactive at the lower temperature than that of the rest catalyst. Owing to the higher reactivity of lattice oxygen, the Ag-doped oxides are catalytically more active in the propylene (C 3 H 6) combustion at low temperature.
3.3.2. NOx removal process
Figure 5 shows that the adsorption of C 3 H 6 and NO x over B was greater than that of A. There are three periods of NO x consumption in the temperature range 120–600 °C from 120 °C, 220 °C and 350 °C over A and 120 °C, 220 °C and 330 °C over B. All three periods started over B at a time earlier than over A. The NO x conversion over B is also higher than that over A. At the relatively low temperatures of 120 °C and 220 °C, an increase in NO x conversion from 23.5% and 29.4% (over A) to 76.5% and 82.4% (over B), respectively, was observed (table 3). The maximum NO x conversion was 82.4% at 220 °C.
The TPSR curve (figure 5) shows that at 120 °C there is the NO consumption without both NO 2 formation and a decrease in C 3 H 6 concentration. In this period, the decrease in NO concentration could relate to the storage of NO or the direct NO decomposition over catalytic centers as oxides MO x-1 by the following reactions:

When a small amount of Ag was added, the silver nanoparticles were well dispersed on the catalytic oxides and played the role of reinforcing the catalytic activity of the material, according to [5], and the metallic silver Ag could participate in the following reaction:

Due to this reaction, the metallic oxide-type MO x-1 was produced incessantly on the surface of the catalyst, and MO x-1 continued to participate in the following reaction:

Combining these factors, we can see that the catalytic reaction of Ag-doped oxides is enhanced in comparison with that of the oxides without Ag doping.
In the two next periods from 220 °C and 330 °C (for B)/350 °C (for A), the NO consumption was observed also without NO 2 formation, but the C 3 H 6 concentration decreased with CO 2 emission at a ratio of near to 1C 3 H 6 → 3CO 2. Hence the samples exhibit both oxidation and DeNO x properties at these temperatures by other mechanisms in comparison with the first period. According to the model of three-function DeNO x reaction [12], the mechanism of 2nd and 3rd periods could be as follows:
In particular, for catalyst B (with Ag-doped), the DeNO x reaction at 220 °C was increased notably. It agreed with [5], whose authors proposed that Ag on a suitable support is able to move NO x with high conversion in the presence of oxygen organic compound C x H y O (as ethanol, acetone ...), which plays the role of reductant. In the present catalytic system, following the schema mentioned above, the 2nd cycle forming C x H y O (from C 3 H 6) is an intermediate product but plays the role of reductant enhancing the 3rd cycle. When the reaction temperature increased above 330 °C or 350 °C, the C 3 H 6 concentration decreased (because of the oxidation reaction), leading to a decrease in C x H y O concentration, hence NO x conversion decreased, as we can see, by 41% and 50% over A and B, respectively. At temperatures above 400 °C, C 3 H 6 was totally converted into CO 2 by oxidation over both catalysts and the DeNO x reaction also stopped.
In summary, the modification of mixed oxides by a small amount (about 1.5–2%) of silver nanoparticles leads to an increase in catalytic activity of material, especially for the DeNO x reaction. The present results are prospective compared with previous publications: the obtained material could catalyze to the DeNO x reaction at low temperature (220 °C) with relatively high NO x conversion as 82.4%.
4. Conclusions
Two catalytic kinds as mixed oxides, A=La 1-x Sr x CoO 3+SrCoO x +SrO and B=A+Ag, were synthesized by the citrate sol–gel method. Most of the particles had sizes ranging from 40 nm to 80 nm.
The metal silver nanoparticles were well dispersed onto the nano mixed oxides but not equally on the surface. The 2% weight of Ag added to the mixed oxides reinforced remarkably the catalytic properties; exceptionally it improved the DeNO x capability of material. The NO x conversion increased from 29.4% (in the absence of Ag) to 82.4% (in the presence of Ag) at the low temperature of 220 °C and the temperature of the total oxidation of propane C 3 H 6 decreased to about 100 °C.
The obtained catalyst is not too expensive but has strong activity at this low temperature. It could be applied in environmental technology for exhaust gas removal.
Acknowledgments
This work was supported in part by Vietnam's National Foundation for Science and Technology Development, No. 103.99-2010.25. The authors are thankful to Professor Acad. Nguyen Van Hieu for his encouragement and interest in this research.