Abstract
This study aims to prepare in manganese ferrite/graphene oxide (MnFe2O4/GO) nanocomposites for the removal of ibuprofen drug residue from water. The synthesised nanocomposites with various MnFe2O4 content from 30–60 wt.% were characterised by X-ray diffraction, Fourier transform infrared spectroscopy, transmission electron microscopy, vibrating sample magnetometer, Brunauer - Emmett - Teller specific surface area, and energy dispersive X-ray. It was found that the suitable mass ratio of MnFe2O4 in nanocomposite for ibuprofen removal was 50 wt.%. The adsorption process followed the pseudo-second-order kinetic and Langmuir isotherm model. The maximum capacity of MnFe2O4/GO was found to be 156.25 mg g−1. Accordingly, the MnFe2O4/GO could be used as an efficient adsorbent for the removal of ibuprofen from water.
Export citation and abstract BibTeX RIS
1. Introduction
Nowadays, residues of pharmaceutical compounds such as non-steroidal anti-inflammatory drugs (NSAIDs) are the most popular groups of pharmaceuticals causing increasingly serious water pollution [1]. Ibuprofen is one of the most widely used NSAIDs and found in detectable high concentrations from wastewater treatment plants [2–4]. The ibuprofen contaminated water has caused serious problems including vomiting, enteritis, mucosal damage, gastric ulcer, the risks of kidney, cardiovascular, and nervous system [5]. Ibuprofen compound is highly degradable and can be degraded well through biological treatment. However, biological processes are expensive, unstable loads, and hard to implement, which is not applied for drinking water. Various technologies encompassing active sludge, adsorption, membranes, photo-catalysts, and oxidation approaches have been developed for the ibuprofen residue removal [6, 7]. Adsorption turns out to be one of the most effective solutions because it is easily recovered, fed continually, low cost, and has capability to adsorb a broad range of removal efficiently [8, 9]. Several adsorbents such as activated carbon, silica, and zeolites have their remarkable capacities to uptake a wide variety of pollutants. The adsorbents are low processing power and difficult to separate and recover after using [10, 11]. Recent studies cited magnetic nano-adsorbent materials such as Fe3O4, NiFe2O4, and MnFe2O4 for many purposes. MnFe2O4 nanoparticles (NPs) with good magnetism and functional surface are widely used as adsorbent. Nevertheless, this material still remains some defects, which include instability and agglomeration which requires it to be attached to substrate surface [12, 13].
Graphene oxide (GO) is oxidised by graphite (Gi) and contained with carboxyl, hydroxyl, carbonyl, and epoxy functional groups which render GO strongly hydrophilic and have good dispersibility in water [11]. Besides, GO possesses highly negative charged density on the surface and large specific surface area to a link of pollutants [6]. These unique properties of GO enable them as ideal substrates to anchor MnFe2O4 NPs for water treatment. The combination of MnFe2O4 NPs and GO to form nanocomposites has the adept points of reusability, easy recovery, and high efficiency for removal of ibuprofen from water. There are two methods of synthesis of MnFe2O4/GO nanocomposites including ex-situ and in-situ (co-precipitation). In ex-situ method, after synthesising MnFe2O4 and GO (precursors), MnFe2O4 NPs are dispersed by using ultrasound onto GO layers. However, the dispersion of MnFe2O4 is distributed flexibly on GO layers [14]. On the other hand, the use of surfactants in nanoparticle synthesis reduces the effectiveness of functional groups and the ability to bind to the substrate. The co-precipitation method is a common technique to synthesise nanocomposite because it is easy to conduct and adjust reaction parameters, time-saving, with more uniform and small size nanoparticles collected [15].
In this work, the MnFe2O4/GO nanocomposites with various mass ratios of oxide were synthesised by co-precipitation method, and applied in removing ibuprofen from water. The characterisation of the nanocomposites was studied by X-ray diffraction (XRD), Fourier transform infrared spectroscopy (FTIR), energy dispersive X-ray (EDX), transmission electron microscopy (TEM), Brunauer - Emmett - Teller specific surface area (BET), and vibrating sample magnetometer (VSM). The selected nanocomposite was investigated in terms of the effects of single factors including pH, contact time, and initial concentration on the adsorption capacity, and calculated for kinetic and isotherm model of removal of ibuprofen from water.
2. Material and methods
2.1. Materials
Graphite (Gi) powder with particle size of 20 μm was purchased from Sigma-Aldrich (Germany). All analytical grade chemicals including H2SO4 (98 wt.%), H3PO4 (85 wt.%), HCl (36 wt.%), FeCl3.6H2O (99 wt.%), MnCl2.4H2O (99 wt.%), H2O2 (30 wt.%), and NaOH (99 wt.%) were purchased from Xilong Chemical, China. KMnO4 and C2H5OH with purities of 99 wt.% were purchased from Vina Chemsol, Vietnam. Ibuprofen (C12H18O2, 99 wt.%) was supplied by Institute of Drug Quality Control – Ho Chi Minh City, Vietnam.
2.2. Methods
GO was fabricated by improved Hummers' method [16]. MnFe2O4 particles and MnFe2O4/GO nanocomposites were synthesised by co-precipitation method [17]. The MnCl2.4H2O and FeCl3.6H2O were dissolved in GO suspension (5 g l−1). Then, the black precipitates were formed by adding each drop of NaOH 2 M in solution to pH 11. The mixture was heated at 80 °C for 2 h under vigorous stirring. The black precipitates were washed with water and dried at 60 °C. The nanocomposites with different mass ratios of 30–60 wt.% MnFe2O4 were marked as MG30, MG40, MG50, and MG60, respectively.
The crystalline structure of materials was analysed on D8 Advance Bruker powder diffractometer with a Cu–Kα excitation source. The functional groups on the surface were studied by FTIR spectrum (Tensor 27, Bruker). The elemental composition was investigated by using scanning electron microscope coupled with energy-disperse X-ray analyser (SEM-EDX) (Jeol JMS 6490, Japan). The morphologies were identified by TEM technique (JEM-1400, Japan) at an accelerating voltage source of 200 kV. The BET specific surface area was measured through N2 adsorption isotherm curve at 77.3 K and po = 765 mmHg (Nova 3200e, Quantachrome). The saturation magnetisation value was recorded using VSM (MicroSense Easy VSM v. 9.13 L).
The effects of MnFe2O4 content, time (15–600 min), pH (3–10), and concentration of ibuprofen (5–480 ppm) on the removal performances of the materials were studied. The conditions were conducted including 20 mg of adsorbent, 20 mL ibuprofen solution, shaking rate of 100 rpm, and room temperature. Finally, the adsorbent was recovered by magnet. The ibuprofen concentration was determined by UV-Vis spectroscopy (Horiba Dual FL, Japan). All experiments were triplicated to estimate the error. The adsorption capacity (q, mg g−1) was calculated following equation (1).
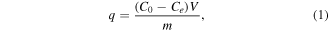
where, Co and Ce (ppm) are the initial and equilibrium concentrations of ibuprofen, respectively; V (mL) is the volume of ibuprofen solution, and m (mg) is the mass of adsorbent.
Besides, the pH values at the potential of zero-point charge (pHzpc) of materials were determined by using the pH drift method. The adsorption data were studied by pseudo-first-order and pseudo-second-order kinetic, Langmuir, and Freundlich isotherm models.
3. Results and discussion
3.1. Characterisation
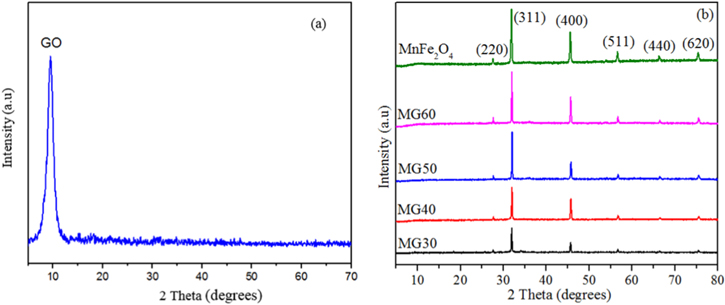
As shown in figure 1, the sharp peak of GO was displayed at 2θ = 11.30°. Gi was oxidised to create GO; and the oxygen-containing groups was inserted into Gi structure. As a result, there was an increase in the distance between Gi layers, which was the basis for Gi's separation of layers to form GO. In particular, the distance of crystalline phase of GO (0.80 nm) is higher than that of Gi (0.34 nm) [18]. Several peaks of MnFe2O4/GO are similar to the cubic spinel ferrite (JCPDS 10-0319) at 2θ = 27.5, 32.5, 46.2, 57.2, 68.4, and 76.5° corresponding to the (220), (311), (400), (511), (440), and (620) planes of MnFe2O4, respectively [19]. The diffraction peak of GO did not exist as shown in figure 1(b) due to the high diffraction intensities of MnFe2O4 NPs overwhelming the weak GO peak. The change in intensities of diffraction peaks in nanocomposites shows the effect of MnFe2O4:GO ratios on the formation of MnFe2O4 crystals. The sizes of MnFe2O4 particles calculated from (311) plane were 15.03, 17.21, 20.15 and 26.35 nm, corresponding to MG30, MG40, MG50, and MG60, respectively. The results revealed the role of GO effect on the creation of MnFe2O4 particles on the surface of the GO sheets.
Figure 1. XRD patterns of (a) GO, (b) MnFe2O4, and MnFe2O4/GO nanocomposites.
Download figure:
Standard image High-resolution imageAs shown in figure 2, FTIR spectra indicated several bands in GO at 3450, 1720, 1640, 1225, and 1080 cm−1 assigned to stretching vibrations of –OH, –C=O, –C=C, –C–OH, –C–O, respectively. This result confirms the oxidation and exfoliation of Gi to create GO. For MnFe2O4/GO nanocomposites, the reduction of the intensities of functional groups indicates that the Mn2+ and Fe3+ ions were linked to –OH, –C=O, and –COOH groups as electronegative positions of GO. The MnFe2O4 NPs are formed at the sites on the GO surface [20]. The elemental analysis of MG50 was confirmed by EDX analysis and shown as table 1. The results indicate that the weight percentage of MnFe2O4 in MG50 is 58.49 wt.%, which is higher than the mass ratio of MnFe2O4 synthesis (50 wt.%) as calculated initially. This is because during the synthesis of a small amount of reduced oxygen-containing functional groups, the ratio of the GO mass in the material sample decreases. This indicates that Mn2+ and Fe3+ are successfully linked to GO surface creating NPs.
Figure 2. FTIR spectra of GO, and MnFe2O4/GO nanocomposites.
Download figure:
Standard image High-resolution imageTable 1. Element analysis by EDX spectrum of MG50.
| Position | C | O | Mn | Fe | MnFe2O4 |
|---|---|---|---|---|---|
| 1 | 21.80 | 35.49 | 14.52 | 28.18 | 58.80 |
| 2 | 21.78 | 35.67 | 35.67 | 27.37 | 58.19 |
| Average | 21.79 | 35.58 | 14.85 | 27.76 | 58.49 |
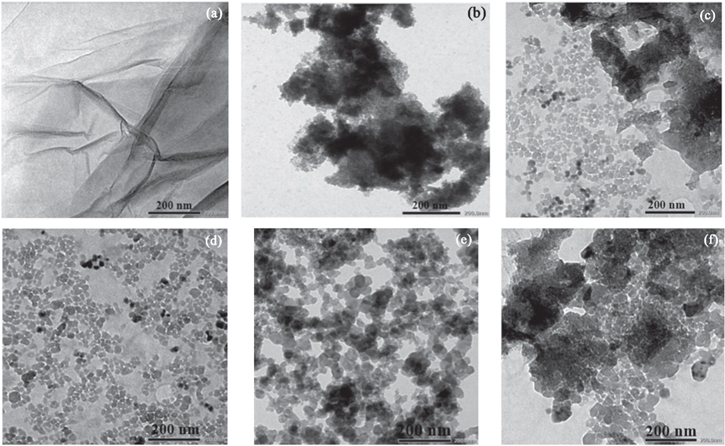
The results revealed the effective combination of GO and MnFe2O4 in nanocomposites, which is consistent with TEM images (figure 3). The MnFe2O4 particles exhibited a nanocluster-shaped morphology, which presented the agglomeration phenomena. For MnFe2O4/GO, the particles were anchored on the surface of GO with the size gradually increasing from 15 to 25 nm. The uniform distribution of particles on the GO accelerates the active sites and surface area of nanocomposite as shown in table 2.
Figure 3. TEM images of (a) GO, (b) MnFe2O4, (c) MG30, (d) MG40, (e) MG50, and (f) MG60.
Download figure:
Standard image High-resolution imageTable 2. The BET surface areas of MnFe2O4/GO and other materials.
| Materials | BET surface areas (m2 g−1) | References |
|---|---|---|
| MG50 | 84.67 | [This study] |
| GO | 62.74 | [This study] |
| MnFe2O4 | 37.80 | [21] |
| MnO2/Fe3O4/GO | 60.10 | [22] |
| NiFe2O4/Graphene | 57.11 | [23] |
These above data showed that the effect of GO substrate helps disperse the formed particles to diminish its size and prevent agglomeration. The oxygen containing groups of GO sheets make the product well-dispersed in aqueous and high chemical activity. These functionalised groups have been negatively charged as anchoring sites for linking the Fe3+ and Mn2+ cations through electrostatic interactions. The MnFe2O4 NPs were formed and deposited on GO sheets via co-precipitation reaction as the following equations (2)–(6). The synthesis process of MnFe2O4/GO was presented in figure 4.
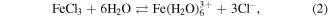
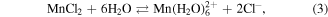
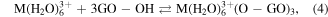
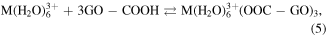
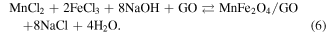
Figure 4. Schematic representation for the synthesis of MnFe2O4/GO.
Download figure:
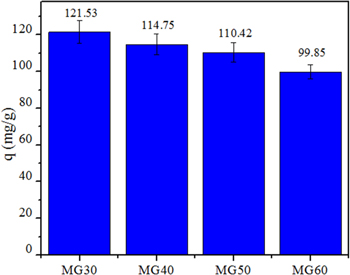
Standard image High-resolution imageWhen further increasing the amount of MnFe2O4, the particles are effortless to link together, reducing the number of active sites and adsorption abilities of materials [24]. Figure 5 shows that adsorption capacities decreased with an increment of MnFe2O4:GO mass ratios. The uptake of MG30 and MG40 was higher than of others materials. However, the lower amount of magnetic oxide makes it difficult to recover by using an external magnetic field [25].
Figure 5. The ibuprofen adsorption abilities of MnFe2O4/GO nanocomposites.
Download figure:
Standard image High-resolution imageThe magnetic properties of MnFe2O4/GO nanocomposites enhanced with an increase in the MnFe2O4 content. The hysteresis loops were examined as shown in figure 6(a) with the curve of S-like shape. The saturation magnetisation (Ms) values increased with an increase in the MnFe2O4 content from 15.93 emu g−1 (MG40) to 20.51 emu g−1 (MG50) whereas the Ms value of MnFe2O4 was 53.18 emu g−1 as shown in table 3. The results are lower than other materials, indicating the reduced size of magnetic particles and non-magnetic ratios in sample [26]. The MnFe2O4 particles were covered by GO sheets in nanocomposites, which also affect the Ms value. MG40 and MG50 were easily separated and recycled by applying an external magnetic field as shown in figure 6(b). The MG50 had a higher percentage of magnetic material, which made easier to recover after adsorption. Thus, MG50 has been recommended as an adsorbent to investigate the effect of factors on ibuprofen removal performance.
Figure 6. VSM result (a), and magnetic properties (b) of MG40, MG50.
Download figure:
Standard image High-resolution image3.2. Adsorption abilities of MnFe2O4/GO nanocomposites
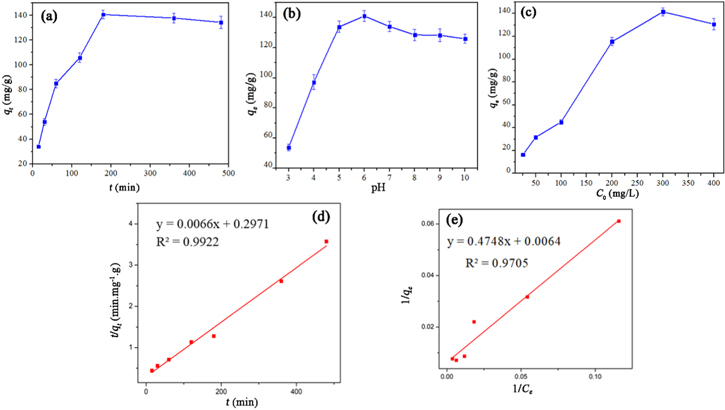
Figure 7(a) showed that the uptake rapidly enhanced with adsorption time and reached the equilibrium after 180 min. Subsequently, the active sites in the MG50 structure were gradually saturated, which leaded to a decrease in the adsorption capacity. The effective time of MG50 for ibuprofen was 180 min. Moreover, the data fitted well with the pseudo-second-order model with the correlation coefficient (R2) of 0.9922 as shown in figure 7(d). Therefore, the adsorption kinetic was mainly affected by the chemical interaction between MG50 adsorbent surface and ibuprofen.
Figure 7. Effect of (a) contact time, (b) pH, (c) initial concentration of ibuprofen, (d) the plot of pseudo-second-order model, and (e) the linear plot of Langmuir isotherm model on the adsorption capacity of MG50.
Download figure:
Standard image High-resolution imageFigure 7(b) shows that when increasing pH, the concentration of H+ in the solution decreased, limiting the competition process between H+ ions and ibuprofen for adsorbent sites, raising the adsorption capacity of MG50 for ibuprofen [6]. The adsorption capacity increased in the range of pH 4 – 6 and reached the highest at pH 6. This was explained that when pKa ibuprofen = 4.33 < pH < pHpzc 5.50, the ibuprofen with positively charged surfaces and the negatively charged oxygen-containing groups on the surface of MG50 (–O−, –COO−) created ibuprofen –COOH2 +, increasing electrostatic interaction and hydrogen bonding between nanocomposite and ibuprofen. However, the adsorption capacity decreased when the pH was higher than 6, due to repulsion interaction between the nanocomposite surface and the ibuprofen group converted into negatively charged anions which create, and reduce the adsorption capacity. The suitable pH for adsorption was determined at pH 6 [2, 11].
Figure 7(c) shows that the uptake correlated with the initial concentration of ibuprofen. The curve went up, then levelled off horizontally, the amount of adsorbed substance increased and then occupied sites in MG50 and obtained saturation state. The adsorption data followed the Langmuir model with a correlation coefficient of nearly 1 (R2 = 0.9857) (figure 7(e)).
And, the maximum adsorption capacity qmax was 156.25 mg g−1, which is higher than other materials in table 4. This was explained due to an advance in the number of adsorption sites to link with ibuprofen when decorating MnFe2O4 on GO [15, 27]
3.3. The mechanism of ibuprofen adsorption
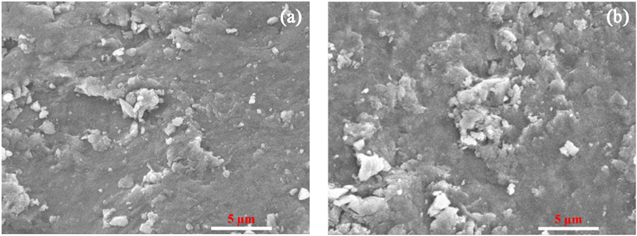
The change of adsorbent after the adsorption process was investigated by using SEM-EDX analysis and FTIR spectra. The SEM image shows that the surface of adsorbent has changed insignificantly after adsorption, indicating the reusable potential of the material in the adsorption field as shown in figure 8. The elemental compositions of MG50 before and after adsorption are shown in table 5. The significant increase of C and O contents after adsorption was higher than before adsorption, which can be explained that ibuprofen was successfully adsorbed onto the surface. Figure 9 shows the FTIR spectra of MG50 before and after ibuprofen adsorption. The intensity of –OH, –C=O, C=C, and –C–O peaks was significant changed, the acidic protons of oxygen-containing groups existing on MG50 surface interacted with ibuprofen due to the appearance of the characteristic peaks of on the spectrum.
Figure 8. SEM images of MG50 before (a), and after (b) ibuprofen adsorption.
Download figure:
Standard image High-resolution imageTable 5. The elemental compositions in MG50 before and after ibuprofen adsorption.
| Element | MG50 | MG50_ ibuprofen |
|---|---|---|
| C | 21.79 | 28.53 |
| O | 35.58 | 37.89 |
| Mn | 14.85 | 11.18 |
| Fe | 27.77 | 22.39 |
Figure 9. FTIR spectra of (b) ibuprofen, MG50 before (a) and after (c) ibuprofen adsorption.
Download figure:
Standard image High-resolution imageIn particular, the ibuprofen removal ability of MnFe2O4/GO was attributed to the electrostatic interaction and hydrogen bonding between the oxygen-containing groups (–OH, –COOH) on the MnFe2O4/GO surface and –OH group in ibuprofen structure. Simultaneously, π-π interaction between the carbon ring of GO and ibuprofen also increases the adsorption capacity [17]. The donor - acceptor mechanism between the oxygen containing groups of GO sheets and the π aromatic ring of ibuprofen was attended in the adsorption process [2].
4. Conclusions
MnFe2O4/GO nanocomposites were successfully synthesised by co-precipitation method with MnFe2O4 mass of 50 wt.% (MG50). The results of characteristic analysis showed that MnFe2O4 NPs were formed and linked on the surface of GO layers with size range from 15 to 25 nm. The ibuprofen adsorption process followed pseudo-second-order kinetic and Langmuir adsorption principle, which might be mono-layer adsorption. The maximum adsorption capacity of MG50 for ibuprofen was 156.25 mg g−1. The MnFe2O4/GO nanocomposite has potential applications in the field of ibuprofen adsorption.
Acknowledgments
This research is funded by Vietnam National University Ho Chi Minh City (VNU-HCM) under grant number C2019-20-31. We acknowledge the support of time and facilities from Ho Chi Minh City University of Technology (HCMUT), VNU-HCM for this study.