Abstract
The green nanoparticles (NPs) are widely accepted as their synthesis is inexpensive and eco-friendly. In the present study, this approach was applied to synthesise nanoscale particles with transition (copper and zinc) and post-transition (iron) metals and aqueous latex of Allamanda cathartica. The selected metals are safe for human use. The experiments were carried out on human peripheral blood mononuclear cells (hPBMCs) isolated from leftover blood sample collected from diagnostic laboratory. The ratio of latex and metal solutions used to prepare NPs was 3% and 1 millimolar respectively. Ultraviolet-visible, Fourier transmission infra-red, scanning and transmission electron microscopy techniques were used to characterise the NPs. The particles were spherical, hexagonal, aggregated and rectangular and the diameter was ranged from 20.5 to 38.6 nm (zinc oxide), 17.9 to 59.2 nm (copper chloride), 29.2 to 55.4 nm (iron sulphate). Fragmentation and/or shearing took place with all the metal NPs, but clear DNA ladder pattern appeared when hPBMCs were treated with latex ZnO NPs. The order of dead cell percentage was CuCl2 (86%) > FeSO4 (87%) > ZnO (95%) after 72 h of treatment with a concentration of 100 μg. These findings indicate that the synthesised NPs are selectively damaging the DNA. Further studies need to be carried out on cancer cell lines to control the cell proliferation.
Export citation and abstract BibTeX RIS
1. Introduction
Nanoparticles (NPs) of less than 100 nm are important measure of nanotechnology. These NPs have various practical applications in fields like agriculture, medicine, biological sciences and so on. Physical and chemical basis of synthesising NPs is widespread to achieve desired characteristics. Considering various limitations, for example, labour involvement, price, impact on biotic and abiotic environment, green approach seems to be advantageous. Synthesis of NPs by green approach is beneficial because of low cost, safety, short time and being easy to scale up [1]. Though many preferred metals are in practice for nanoparticle synthesis, one has to choose cheapest metal with high possible benefits. Among those, zinc oxide (ZnO) NPs' properties have been expanded in such a way that they are safe to use for drug delivery, diagnostics of various diseases, bacterial infections, skin issues such as burns and wounds. Metal ions are generally considered as centers of anticancer agents [2, 3]. One among the main subdivisions of bioinorganic chemistry is the introduction of metal ions or compounds into living systems to treat various diseases or disorders [4]. There are well explained synchronisation geometries between reversible recognition of nucleic acid research and transition metal centers of particular attractive moieties. Since last decade, copper chloride, zinc oxide and ferrous oxide, which occupy transition and post-transition position in periodic table, paved a way for their use in synthesising NPs. They have good applications in consumer products, environmental remediation, personal care, biomedical, food packaging and so on [5]. Yet, there is an urgent need to use cheaper first line compounds binding to DNA efficiently with cytotoxic activity. Therefore, the focus of research is on pharmacological importance of cheapest and rapidly available first line metal compounds viz. copper, zinc, and iron complexes. Additionally, these metals have an essential place in intracellular systems of living organisms and are co-factors for over 300 proteins of the mammalian system. ZnO is widely used in cosmetics, soaps, lotions, nail polishes, skin protectants and diaper rash ointments. Food and Drug Administration has also approved skin protectants with a permitting level of 25% ZnO [6]. NPs made with this metal are eco-friendly, non-toxic and biologically safe. When mixed with plant material, they form ZnO green NPs, which makes them ideal candidates for biological applications [7]. ZnO NPs show activity on microbes in food packaging [8], UV-absorbing agents and in personal care [9]. ZnO successfully reduces colon cancer cell viability and has been used as drug transporter for targeted treatment in leukemia and hepatocarcinoma cells [10]. Plant based ZnO NPs were tested as antibacterial, antidiabetic, photocatalytic, and antioxidant agents. Research claims that ZnO NPs are free of harsh chemicals and clean, making them suitable for pharmaceutical, biological and medical applications. Copper based NPs click a reaction of plant based azides and terminal alkynes to synthesise NPs. Advantageous side of copper in synthesising NPs is its low cost and abundance in availability, on the other side, toxic chemical usage during Cu NPs synthesis limits its use in clinical applications [10]. Green synthesis limits the generation of toxic chemicals. In any NPs' synthesis with metals, the three important factors are the solvent medium, reducing agent and capping agent. Plants and their derivatives contain primary and secondary metabolites which act as reducing and/or capping agents. Iron sulfate or ferrous sulfate exists as heptahydrate. In medical field, the hydrated form is used as treatment for iron deficiency to treat anemia. As per the World Health Organisation, it is one among the essential medicines listed in basic health system. It is an excellent reducing agent, used to fortify foods [11]. It is known that Fe NPs combat environmental pollution with promising advantages. Also, the nanoscale iron has large surface to volume ratio. The unlimited step in the nanomaterial improvement is the synthesis of iron nano materials via green route [12, 13]. There is a huge demand for plant based products in clinic, agriculture and therapeutics. Plants harbour many active compounds with therapeutic value. Herbal medicine purely depends upon the overall function of these active compounds. Herbal originated drugs also have a few limitations in direct usage. In recent times, use of plant natural products has increased tremendously. A few plants produce latex as defence element, an amazing source of biomolecules such as glycosides, tannins, saponins, phytosterols, to name a few. The presence of phytochemcials makes the latex fight with microbes, nematodes, insects and markedly cancers [14]. Evidently, all the plants belonging to Apocynaceae produce latex and one among those is Allamanda cathartica L. As the diameter (primary) of natural latex ranges between 100 and 300 nm, it is considered as a promising start material for preparing NPs which can be used in medical and pharmaceutical applications. Silver NPs made with aqueous latex showed dose dependent antiproliferative activity on human peripheral blood mononuclear cells (hPBMCs) [1, 15–17].
Considering the advantages of using latex, we are interested in showing the comparative effectiveness of transition metal and post-transition metal NPs synthesised using aqueous latex obtained from A. cathartica on hPBMCs. The study would be novel as many published articles represented independent metal's results for making NPs from plant extracts, but there is no comparative analysis among the chosen metals and latex of the selected plant in the present study. Furthermore, studies showed that higher concentrations of NPs (1 to 100 mg ml−1) showed cytotoxicity [18]. In this study, for checking the in vitro cytocompatibility of the lowest concentration (100 μg ml−1) of NPs, we used hPBMCs and demonstrated the DNA ladder pattern.
2. Materials and methods
Allamanda cathartica plant was collected from Sri Devaraj Urs Medical College garden, Kolar, identified and authenticated by Horticulture College, Kolar. Latex was collected in the morning hours from the trimmed young twigs and stored at 4 °C till NPs synthesis. Phytochemical analysis of aqueous latex (3 mg wet weight per milliliter water) was carried out by Harbourne's protocols [19].
2.1. Phytochemical analysis
2.1.1. Test for alkaloids
About 200 μL of aqueous latex was added to 2% warm sulfuric acid for two min followed by adding a few drops of Dragendorff's reagent. Orange colour precipitate indicates the presence of alkaloids.
2.1.2. Test for flavonoids
To 200 μL of aqueous latex, a few drops of 10% lead acetate and 5 ml of distilled water were added and vortexed well. Formation of deep yellow colour indicates the presence of flavonoids.
2.1.3. Test for saponins
Aqueous latex (200 μL) and distilled water (5 ml) were mixed vigorously and boiled to see the froth.
2.1.4. Test for tannins
Ferric chloride was added to latex solution to get dark green solution.
2.1.5. Test for glycosides
The latex solution was first mixed with HCl, NaOH solution was added to make it neutral and finally a few drops of Fehling solution were added for the development of colour.
2.1.6. Test for reducing sugars
Aqueous latex was treated with Benedict's reagent and heated gently. If the colour changes to blue, there is no presence of sugars and if the colour changes to green, yellow, orange, red or brown, it indicates the presence of sugars.
2.2. Nanoparticle synthesis
Equal volumes of 1 mM metal solution (Cu, Zn and Fe) were mixed properly with 3% water soluble latex solution and the mixture was incubated at 37 °C in an incubator shaker (135 rpm) for 24 h. The development of colour was observed and the samples were stored at 4 °C [17].
2.3. Latex nanoparticle characterisation
To check the absorption spectra of the synthesised NPs, UV-visible spectrophotometer was used. Particle spectra were obtained from Nanodrop 8000 between 200 and 700 nm. The gold standard methods for characterisation of NPs are scanning electron microscopy (SEM) and transmission electron microscopy (TEM). The preparation protocol followed [16, 17], with a monor modification. Briefly, 2 ml of green NP was centrifuged at 4000 rpm for 20 min. The dried pellet was scrapped carefully with forceps and placed onto a glass slide. The images were captured in a SEM, Quanta 200 (FEI) and carbon coated copper 300 mesh grids were used for TEM analysis. About 10 μL green metal NPs was placed on metal coated copper grids which were dried under lamp. Tecnai G2 Sprit Bio TWIN with a voltage of 120 kV was used to obtain the images. The X-ray Diffraction (XRD) measurements were recorded on Bragg angle 2θ with 2/min scanning rate. This was done on a spectroscope by using Seifert Rayflex 300TT X-ray diffractometer with CuK (λ = 1.542 Å) radiation that was operated at a voltage of 40 kV and a current of 30 mA with Cu k-α radiation (1.5405 Å). To disclose the involved organic functional groups on the NPs, Fourier Transform Infrared spectroscopy (FTIR) was performed on Nicolet 6700 FTIR. Method involved was potassium bromide pellet and the range of the scan was from 4000 cm−1 to 400 cm−1. SEM, TEM, FTRI and XRD were done in Department of Microbiology and Cell Biology (MCBL), Spectroscopy Analytical Test Facility, and Materials Research Centre (MRC), Indian Institute of Science, Bengaluru.
2.4. Collection and incubation of hPBMCs with green NPs
Central ethics clearance was (SDUAHER/KRL/Res. Project/128/2017-18) obtained from the institute before we start the isolation of hPBMCs from leftover blood (collected in heparin tubes) from Central Diagnostic Laboratory, Sri Devaraj Urs Academy of Higher Education and Research. Without any modification, Ficoll gradient method was followed (1). hPBMCs were allowed to grow in RPMI for 4 h and inoculated with 20, 40, 60, 80 and 100 μg/ml green NPs from 1 mg ml−1 stock. Minimum and maximum concentrations of DMSO, metal and 3% latex were used as controls. To name the controls: only hPBMC, hPBMC + DMSO (20 μL), hPBMC + DMSO (100 μL), hPBMC + 1 mM metal solution (20 μL) hPBMC + 1 mM metal solution (Cu, Zn and Fe) (100 μL), hPBMC + 3% latex (20 μL) hPBMC + 3% latex (100 μL).
2.5. Trypan blue dye exclusion
Trypan blue (0.4%) in balanced salt solution and hPBMCs suspension were mixed in equal volumes and undisturbed for about 10 min at room temperature. About 10 μl of this suspension was transferred onto a clean slide and the cells were counted in hemocytometer [20].
2.6. DNA ladder pattern of hPBMCs
DNA ladder pattern of hPBMCs was noted by taking 104 cells and lysed with TES buffer (1 M Tris, 20 mM EDTA and 1% SDS) [21]. To the clarified cell pellet, 20 μL of lysis buffer was added and mixed properly, 10 μL RNase was added to the lysed cells, and left for one hour at 37 °C. The temperature was shifted to 50 °C, 10 μL Proteinase K was added and incubated for 1.5 h. The entire volume was loaded in 1% agarose gel with 6X DNA loading dye and ran at 80 to 100 volts. Image was documented by UV-transilluminator.
2.7. Statistical analysis
All the experiments were repeated three times to minimise the error and standard errors were calculated.
3. Results and discussion
Copper NPs have similar properties and challenges as noble metals such as gold, silver and palladium. As a general statement, noble metals resist oxidation, corrosion, and are not easily attacked by acids. Cu and Cu NPs are cheaper when compared to other metals.
Mainly, flavonoids, saponins, tannins reduce and chelate the metals. The functional groups of the flavonoids reduce the copper ion. The assumption is that in the process of making Cu NPs, a reactive hydrogen atom of the flavonoids is released and reduces the copper ions to form copper nuclei or Cu NPs. In addition to flavonoids, other phytochemicals viz, alkaloids, saponins, glycosides, carbohydrates and lignins also act as reducing and stabilising agents [22].
Studies proved that metallic NPs in particular, iron NPs (Fe NPs) have capacity to fight environmental pollution. Iron in nanoscale is a zero-valent and this property makes it to have large surface area to volume ratio [12, 13]. This magnetism disappears when the magnetic field is removed at elevated temperatures. Because of the magnetic properties, Fe NPs are useful for various applications [15]. Prasad et al used Garlic vine and FeSO4.7H2O for preparing Fe NPs [23]. At room temperature or in an outer magnetic field, magnetic NPs, having smaller diameter, display high capacity magnetism [24, 25]. Based on this property, we used room temperature for preparing Fe NPs. To test and compare the effectiveness, in the present study we used metals as nano-carriers, Allamanada latex as phytochemical source on hPBMCs.
3.1. Phytochemical screening
Tests for phytochemicals revealed that the aqueous latex has the following phytochemicals: flavonoids (yellow), saponins (frothing), glycosides (red) and reducing sugars (orange red).
3.2. UV-visible
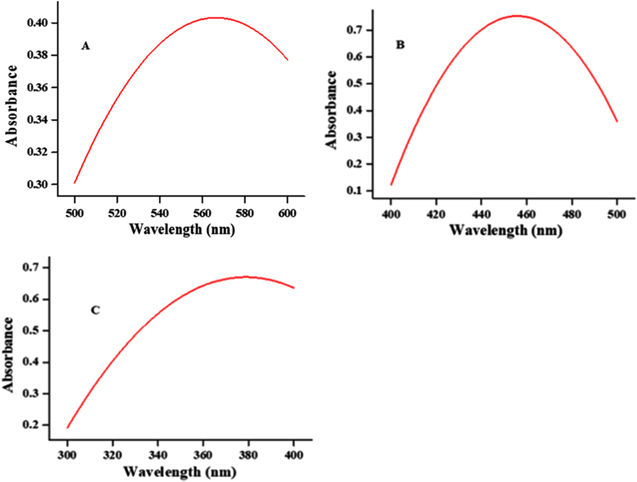
Latex NPs were made by mixing equal volumes of 3% aqueous latex with 1 mM transition (copper chloride and zinc oxide) and post-transition (iron sulphate) metal solutions. Overnight incubation developed the colours, viz, dark brown, greenish black and bottle green with iron sulphate, copper chloride and zinc oxide respectively (figure 1). This indicated the excitation of surface plasmon resonance (SRP). Latex phytochemicals are responsible for the formation of NPs [26].
Figure 1. Change in colour of the solution when latex (3%) and metal (1 mM) solutions were mixed. From left: Cu NPs, Zn NPs and Fe NPs.
Download figure:
Standard image High-resolution imageFigure 2 gives the information about the UV-visible spectrum of NPs wherein, the absorption maximum was perceived at 575, 450 and 370 nm for copper, iron and zinc respectively. A study conducted by [27] showed the same peak pattern for zinc, [28] copper and [29] iron. The light coloured mixtures changed to dark colours, which is indicative of formation of oxide particles by irreversible interaction between phytochemicals and metals.
Figure 2. UV-visible spectra of copper (A), iron (B) and zinc (C) NPs at their absorption maxima.
Download figure:
Standard image High-resolution image3.3. SEM and TEM
The morphology, surface and size details of metal latex NPs were studied by SEM and TEM. The advantage of taking both the microscopies into consideration is that both will provide similar but distinct analysis. TEM images help us to know the size and shape of the synthesised NPs. By TEM, the captured particles were 21; the particle diameter ranged from 17.9 to 59.2 nm. The average size of the particle is 33.5 nm. Besides, the synthesised NPs are mostly round or spherical, a few were aggregated, having a wide-range size dispersal and figure 3 shows the TEM and SEM images of the particles. The results are in accordance with Viet et al [30]. Latex phytochemicals offer flexible control over the size and shape of the NPs. In NP biosynthesis, phytochemicals first reduce the metal ions and stabilise the metals in the form of NPs. So it is thumb of rule that phytochemicals of the latex control the size and shape of NPs. Results showed that the synthesised NPs are mostly round or spherical, hexagonal, aggregated and rectangular below 59, 38.6 and 55 nm for Cu NPs, Zn NPs and Fe NPs respectively (figure 3).
Figure 3. TEM images of metal NPs synthesised after mixing metals and latex. A. Cu NPs at magnification of 100 nm. B. Fe NPs at magnification between 100 and 200 nm. C. Zn NPs at magnification between 200 and 500 nm. Lower panel shows the images of the SEM of the particles.
Download figure:
Standard image High-resolution imageThe shape of the NPs was noted as spherical, rectangular, irregular, angular, hexagonal and aggregated. The NPs diameter ranged from 17.9 to 59.2 nm (Cu NPs); 20.5 to 38.6 nm (Zn NPs) and 29.2 to 55.4 nm (Fe NPs).
3.4. FTIR spectroscopy
In general, FTIR spectroscopy analyses chemical composition of biological and non-biological materials. This analysis provides both qualitative and quantitative measures of material. The compounds present in test sample form a cap to metals thus stabilising the metal NPs. This method helps to identify the likely interactions between the metal NPs and test sample, in our study, the latex. IR spectra are the signatures of amide linkages of proteins and polypeptides. Figure 4 shows the FTIR spectra of the NPs taken between 4000 and 400 cm−1. The present study results reveal different stretches of bonds at different peaks.
Figure 4. FTIR spectra of the NPs between 4000 and 400 cm−1. Copper, iron and zinc NPs were characterised by FTIR and the respective peaks were described and confirmed as NPs.
Download figure:
Standard image High-resolution imageIn figure 4, Cu NPs FTIR spectrum, a broad peak at 3175.61 cm−1 presents O-H stretch, strong and broad and compound class is carboxylic acid. Peak at 2922.49 cm-1 might be carboxylic acid O-H stretch, −C–H stretch medium appearance and compound class is alkane; 2850.15 cm−1 peak might be carboxylic acid O-H stretch, medium –C-H stretch, alkane; peaks at 2359.57 cm−1 and 2337.23 cm−1 are strong, O=C=O stretch carbon dioxide, carboxylic acid O-H stretch; peak at 1731.46 cm−1 might be strong, C=O stretch aldehyde, aldehyde C=O stretch; and 1402.47 cm−1 peak might be strong, C-F stretch fluoro compound. FTIR spectrum of FeNPs is presented in figure 4. Peak at 3272.48 cm−1 is strong, broad, O-H stretch, alcohol/phenol, carboxylic acid; 2922.67 cm−1 peak might be medium C-H stretch alkane/alkyl, carboxylic acid O-H stretch; peak at 2850.64 cm−1 is medium C-H stretch alkane, carboxylic acid O=H stretch; and 2357.30 cm−1 peak might be strong, O=C=O stretch, carbon dioxide. FTIR spectrum of Zn NPs shows peak at 3176.24 cm−1, a strong, broad O-H stretching carboxylic acid; 2922.12 cm−1 peak is a strong, broad N-H stretch amine salt, C-H alkyl stretch; peak at 2849.97 cm−1 might be medium, C-H stretching, alkane and alkyl stretch; 2357.80 cm−1 peak is a strong, O=C=O stretching, carbon dioxide and 1730.53 cm−1 peak is a strong, aldehyde C=O stretch, α, β-unsaturated ester. The results are in agreement with a study conducted by using Euphorbia jatropha latex [27].
3.5. Cytotoxicity of NPs on hPBMCs
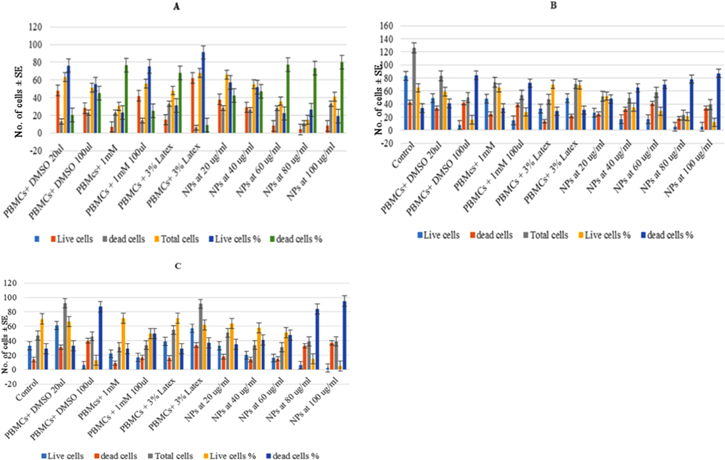
To test the effectiveness of the synthesised NPs as nano-carrier antiproliferative drugs, we tested the cytotoxic property of these NPs in terms of DNA damage, hPBMCs were exposed to different controls (positive and negative), and treatments. Cytotoxicity of NPs was carried out on hPBMCs with controls, NPs dose range was between 20 and 100 μg ml−1, replicated three times and the results were expressed as mean ± S.D. The synthesised NPs were made to 1 mg ml−1, the hPBMCs were treated at 20, 40, 60, 80 and 100 μg ml−1, the minimum (20 μg ml-1) and maximum concentrations (100 μg ml−1) of DMSO, latex and metal were treated and incubated for 72 h. All the controls and treatments were stained with trypan blue to differentiate live and dead cells. In all the experiments, we observed a dose depended response, which means that as the NPs' concentration increased, there is an increased percentage of dead cells and decreased percentage of viable cells. Images were captured using CARL ZEISS microscope (Axion imager A2). Figure 5 represents the percentage of viable and dead cells obtained from respective treatments and controls. The order of antiproliferative activity of the NPs was Cu (86%)>Fe (87%)>Zn (95%). In-depth investigations are needed to find the mode of action of NPs (figure 5).
Figure 5. Percentage of viable and non-viable hPBMCs after treatment with increased concentrations of copper (A), iron (B) and zinc (C) NPs. Values were represented as mean ± SE.
Download figure:
Standard image High-resolution image3.6. DNA ladder pattern of hPBMCs
Researchers are focusing on interaction between small molecules and DNA. One of the primary intracellular targets of anticancer drugs is the DNA of any cell type and interaction between them causes DNA damage by blocking cell division and leads to cell death [31]. Enthusiastically many researchers interested in finding out the binding properties of NPs to DNA, as new cancer drugs [32, 33]. The qualitative evaluation of NPs is the DNA fragmentation (figure 6).
Figure 6. Agarose gel pictures showing DNA fragmentation pattern of hPBMCs treated with latex NPs. NPs were prepared by mixing 3% latex and 1 mM metals. Image showed clear fragmentation of the DNA with Cu NPs (A), Fe NPs (B) Zn NPs (C).
Download figure:
Standard image High-resolution imageControl hPBMCs in A, B and C were free of any treatment, the DNA is still seen in the well, since there is no genotoxicity, indication of undamaged DNA, where as in other lanes, though there is no shearing of DNA, fragmentation was noted. Similar results were noted when MCF-7 cells were treated with Cu NPs at 160 and 320 μg ml−1 doses, but comparatively, the NPs concentration was lesser (maximum 100 μg ml−1) in our study [34].
In this study, equal volumes of 1 mM metal solution and 3% water soluble latex solutions were mixed properly at 37 °C in an incubator shaker (135 rpm) for 24 h to synthesise NPs. The change in colour indicates the formation of NPs. The NPs were characterised by standard methods and their efficacy was tested against hPBMCs. Barar et al in 2015 prepared metal NPs by using anticancer drug, mitoxantrone (MTX) [35]. The maximum particle size by TEM was 35 nm, wherein, the NPs synthesised by us also showed an average particle size of 34.6 nm. The smaller NPs of <10 nm of any metal can be removed quickly from the blood stream whereas larger particles are cleared by mononuclear phagocyte system. In our study we used hPBMCs, wherein if these cells are under uncontrolled condition, when given NP treatment, because of their nanosize, NPs can eliminate the diseased cells quickly from the blood stream to kidney to remove from the body. In other words, particle size and penetration go hand in hand; accumulation with the tumour is higher when smaller NPs interact with tumour. Alone, Cu metal at both the concentrations showed less toxicity to the cells, indicating the safe use of Cu metal in synthesising the NPs. The capping phytochemicals make Cu more effective in controlling the proliferation of hPBMCs, where the NPs can be used to control the division of lymphocytes in leukaemia. The mechanism of action of NPs on hPBMCs and non-effectiveness on normal cells with minimum side effects has to be elucidated. Though the NPs are non-toxic, latex is more effective in making the NPs more stable and biocompatible. Among the used transition and post-transition metals and metal oxides, it is evident that Zn NPs when mixed with Allamanda latex showed 95% effective antiproliferation property. Zinc, the 2nd most transition metal, is considered to be an essential micronutrient required for the metabolic activities of humans, animals and plants [28]. Zn NPs can also be used for selective destruction of tumour cells and have a great potential in drug delivery applications [36]. ZnO-NPs have also been shown to exhibit strong protein adsorption properties, which can be used to modulate cytotoxicity, metabolism or other cellular responses [37]. Apart from these many applications, ZnO, due to its low toxicity, is listed as 'Generally Recognised as Safe' (GRAS) by the US Food and Drug Administration (21, CFR 182, 8991). In several studies it is evidenced that Zn NPs showed an increased in vitro cytotoxicity in glioma, colon, bone, breast, leukaemias and lymphomas [38–40]. Cancerous cells of lymphatic family when matched with the normal cells were approximately 28 to 35 times more vulnerable to Zn NPs mediated cytotoxicity [39, 41].
It is discussed by Rasmussen et al in 2010 [36] that Zn NPs can be a source of selective destruction of cancer cells and also have unlimited prospects in drug delivery applications. Modulation of cytotoxicity is due to ZnO NP's strong protein adsorption properties [42]. Ravindra et al in 2011 used Calotropis procera latex and ZnO for NPs synthesis and the average particle size was noted between 5 and 40 nm [43] and the maximum NPs in our works were 31 nm. Effect of ZnO NPs at a concentration of 1000 μg ml−1 against PBMCs was determined by Rashmirekha Pati et al, [18] and clarified that ZnO NPs are significantly less, which is 15% reduction in cell viability. In our study, about 95% cells were dead when treated with a highest concentration of only 100 μg ml-1 [18].
4. Conclusion
The transition and post-transition metals and Allamanda, a medicinal plant, latex were used to synthesise NPs by inexpensive and eco-friendly route. The particle diameter ranged from 20.5 to 38.6 nm (zinc oxide); 17.9 to 59.2 nm (copper chloride); 29.2 to 55.4 nm (iron sulphate). Fragmentation and/or shearing were observed with all the metal NPs, but clear DNA damage/ladder pattern appeared when hPBMCs were treated with latex Zn NPs.
To conclude, the biologically synthesised NPs can be selective in damaging DNA of hPBMCs. Further analysis can be carried out by using cancer cell lines by performing scientifically approved techniques to provide concrete evidence to control and target cancer cells.
Acknowledgments
We sincerely acknowledge the financial assistance from Sri Devaraj Urs Academy of Higher Education and Research, Kolar, Karnataka, India. We acknowledge the Department of Microbiology and Cell Biology (MCBL), Spectroscopy Analytical Test Facility, and Materials Research Centre (MRC), Indian Institute of Science, Bengaluru, Karnataka, India for their help in characterisation of NPs.