Abstract
Titanium dioxide nanotubes (TNTs) are synthesised by hydrothermal method. Characterisations of TNTs are surveyed by transmission electron microscopy (TEM) images, x-ray photoelectron spectroscopy (XPS), and the Brunauer–Emmett–Teller (BET). The adsorption capacity and photocatalytic degradation of TNTs toward methyl blue (MB) are investigated under different pH values from 1 to 12. The results demonstrated that medium pH affected the charged state of hydrothermally synthesised TNTs surface, the adsorption capacity and photodegradation activity of TNTs. The study indicated that the adsorption capacity of hydrothermally synthesised TNTs is strongest at pH 6 and weakest at pH 1. In addition, the MB photocatalytic degradation of TNTs exhibited the highest efficiency of 86.87% at pH 5 and the lowest one of 42.24% at pH 1 after 300 min under UV irradiation.
Export citation and abstract BibTeX RIS
1. Introduction
The problem of environmental pollution is becoming more and more serious such as municipal wastewater, dying, printing, textile, domestic sewage, which has a serious influence on our lives and has attracted our attentions [1]. Recently, the achievements in nanotechnology field have indicated that nanomaterials have great potential in the water treatment by the removal of phenol compounds, dyes from industrial wastewater through the adsorption and photocatalytic processes [2–8]. At nanoscale, materials behave very differently in their physical, chemical or biological properties, especially, under photoirradiation [9, 10]. In the industrial wastewater treatment, materials are often used to adsorb or photodegrade some dyes or potentially toxic metals [11–13]. Among various materials at nanoscale, TiO2 nanotubes (TNTs), have attracted tremendous scientific and technological interest owing to the large specific surface area, high ion exchange capacity, and chemical stability, economic viability [14–16]. Moreover, they are an eco-friendly material, can be synthesised by simple method with low cost [17, 18]. There are many factors affecting the photocatalytic applications of the TNTs such as the dye concentration, the amount of catalyst, the pH of the medium, and the synthesis method, etc [5, 19, 20]. Literally, the pH of the medium is one of the most important parameters for the photocatalytic activity because of its effect on the photooxidation, and electrostatic attractive effects between the charged surface of photocatalyst and pollutant molecules leading to the change of the adsorption ability of the materials [21–23]. Iraj et al [24] indicated that the adsorption of the dyes onto ZnO nanoparticles surface is strongly dependent on the pH value of the reaction solution and it is an important step in photocatalytic degradation. In the review of Akpan et al [25], some dyes are photocatalytically degraded at lower pH, while others are only degraded at higher pH value. The published studies only investigated individually pH values [21, 26–28]. In addition, pH values in the wastewater are often high (about 11–12) [29, 30]. Therefore, the determination of the probably right pH to thoroughly photodegrade the nature of the pollutants by using TNTs is important to study.
In this study, TNTs are synthesised by hydrothermal method, and then, the investigation of the methylene blue (MB) adsorption of hydrothermally synthesised TNTs is conducted at wide pH value range, from 1 to 12. Moreover, the relationship of the adsorption and photocatalytic activity of hydrothermally synthesised TNTs under UV irradiation is also surveyed at different pH values.
2. Experimental
2.1. Materials and chemicals
Commercial TiO2 powder (Merck, 99.99%) with microscale, MB were obtained from JHD Fine Chemicals (China, 99%), sodium hydroxide (NaOH, Merck, 99%) and hydrochloric acid (HCl, Merck, 99.99%). Deionised water was supplied by Puris-Evo water system and used in all experiments.
2.2. Preparation of TiO2 nanotubes
TNTs were prepared by the hydrothermal method at the optimised synthesis parameters [18]. Briefly, approximately 1.7 g commercial TiO2 powders was added to 157 ml NaOH 10 M solution and stirred for an hour at the room temperature. Next, the mixture was placed in an autoclave and heated at 135 °C for 24 h. After the hydrothermal process, the resultant white precipitates were subsequently filtered and repeatedly washed with acid HCl 0.1 M and deionised water until the pH of the filtrate reached approximately 7.0. The obtained TNTs were dried in air at 80 °C for 6 h and stored in airtight brown bottles until required for use in the following experiments.
2.3. Characterisations of TiO2 nanotubes
The phase, composition, and crystal structure of the materials were determined by x-ray diffraction (XRD) pattern, using a Bruker D8-Advance 5005 with Cu-Kα radiation (λ = 0.154064 nm). The morphology of hydrothermally synthesised TNTs was observed by transmission electron microscopy (TEM) images using a transmission electron microscope on a JEM 1400 instrument, JEOL. The chemical state of titanium, and oxygen in materials were analysed by x-ray photoelectron spectroscopy (XPS) using a Leybold spectrometer with an Al Kα monochromatic beam (1486.6 eV, ESCALAB250, Theta Probe XPS system). The specific surface area (SBET) was calculated from nitrogen adsorption/desorption using the Brunauer–Emmett–Teller (BET) equation, and the pore distribution was determined by the Barrett–Joyner–Halenda equation. Before analysing textural property, the sample was degassed at 120 °C for 2 h. The photocatalytic activity of the material was investigated using an UV–vis spectrometer (U2910, HITACHI, Japan) with the wavelength in the 400–800 nm range.
2.4. Dye adsorption experiment
Firstly, MB solution was adjusted into target pH value by adding 1 M NaOH or 1 M HCl solutions. Secondly, approximately 0.02 g TNTs was introduced and magnetically stirred with 60 ml MB (20 mg l−1) into the closed system with different pH values. The adsorption process was conducted in a dark condition. Thirdly, the solution was centrifuged and the absorption spectrum was calculated to measure the MB concentration. Finally, this solution was poured back into the initial solution and continuously stirred in the dark.
2.5. Dye photodegradation experiment
After measuring the adsorption ability of the sample for 60 min in the dark, the MB solution was irradiated under UV light (7 W, λ = 350 nm). The mixture continued to be stirred throughout the irradiation. The absorbance of the sample is determined every 30 min with a maximum absorbance peak (λmax = 664 nm) for each sample. The photodegradation efficiency (η%) is calculated as follows:
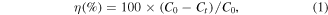
where C0 and Ct were the concentrations of initial MB after t min of UV light exposure, respectively.
3. Results and discussion
3.1. Characterisation of TiO2 nanotubes
Figure 1(a) presents the morphology of TNTs synthesised by the hydrothermal method. As expected, the synthesised TNTs exhibited a relatively homogeneous size with its length from 200 to 400 nm, average outer diameter 10 ± 2 nm, and average inner diameter 6 ± 2 nm. Figure 1(b) shows HRTEM image of hydrothermally synthesised TNTs, therein, the measurement on lattice spacing (dspacing) in the micrographs confirmed the crystallisation of hydrothermally synthesised TNTs in the anatase phase with d = 0.352 nm, and 0.238 nm corresponding to (101) and (004) planes, respectively [31]. The result demonstrated the formation of the TiO2 crystals.
Figure 1. TEM (a) and HR-TEM (b) images of hydrothermally synthesised TNTs.
Download figure:
Standard image High-resolution imageXRD result for the commercial TiO2 and hydrothermally synthesised TNTs is given in figure 2. As expected, all studied materials exhibited characteristic diffraction peaks at 2θ of 25.25°, 37.72°, 47.89°, 54.43°, 55.03°, 62.83°, and 68.82° corresponding to the (101), (004), (200), (105), (211), (204), and (116) crystal lattice planes of the anatase phase of TiO2, respectively (JCPDS no. 21-1272). Moreover, the presence of diffraction peaks at 2θ = 27.5° and 2θ = 36.14° is attributed to the (110) and (101) crystal lattice planes of the rutile phase of TiO2, respectively (JCPDS card no. 21-1276). The formation of the anatase phase and the rutile phase indicated the enhancement of the photocatalytic activity for TNTs [32].
Figure 2. XRD patterns of the commercial TiO2 and hydrothermally synthesised TNTs.
Download figure:
Standard image High-resolution imageElemental oxidation state was determined by a high-resolution x-ray photoelectron spectroscopy (HRXPS). Clearly, figure 3 shows that two biding energy (BE) peaks in HRXPS of Ti 2p orbital at 464.3 and 458.59 eV were assigned to Ti 2p1/2 and Ti 2p3/2 of Ti4+ in TiO2, respectively [33, 34]. In addition, HRXPS spectrum of O 1 s confirms the existence of two states with BE at 532.5 eV and 530.2 eV corresponding to O2− states in TiO2 [34]. The HRTEM and XRD results are consistent with the XPS result. Therefore, it can be concluded that TNTs were formed with high crystallisation and an excellent complex.
Figure 3. HR-XPS spectra of (a) Ti 2p and (b) O 1 s.
Download figure:
Standard image High-resolution imageThe textural property of hydrothermally synthesised TNTs and commercial TiO2 has been published in our previous study [18]. The Brunauer–Emmett–Teller surface area of hydrothermally synthesised TNTs (83.49 m2 g−1) was significantly higher than that of commercial TiO2 (40 m2 g−1, a non-porous material). An analogous result was obtained by Wang et al, Pai et al [35, 36] and Son et al [37] who reported that BET surface area of commercial P-25 TiO2 Degussa is 56 m2 g−1 and 50 m2 g−1, respectively. In addition, TNTs can be classified as a mesoporous material because of its pore diameter approximately 4.08 nm (in 2–50 nm range). The result suggested that TNTs possibly remove MB cations from water media through the adsorption and photocatalysis processes.
3.2. The relationship of adsorption ability and photocatalytic activity of TiO2 nanotubes
The MB adsorption capacity and photodegradation efficiency of hydrothermally synthesised TNTs are experimented in different pH values. The effects of solution pH (pHsolution) on the process of MB adsorption onto TNTs are provided in figure 4. The point at zero charge (pHpzc) of hydrothermally synthesised TNTs determined by the drift method was approximately 6.0, which is consistent with the findings of other scholars, such as pHpzc of hydrothermally synthesised TNTs = 6.25 [38].
Figure 4. MB adsorption efficiency of hydrothermally synthesised TNTs for the medium with different pH values.
Download figure:
Standard image High-resolution imageEssentially, when pHsolution > pHpzc, the surface of hydrothermally synthesised TNTs will become negatively charged because of the deprotonation of –OH groups (equation (2)), favouring the adsorption of MB cations from the solution and vice versa [28, 39]. Clearly, the adsorption capacity of MB onto TNTs was negligible when solution pH was lower than 2.0 due to the presence of strong repulsion force (equation (3)). However, the adsorption capacity of hydrothermally synthesised TNTs toward MB increased with an increase in solution pH, with its highest adsorption capacity at pH 6.0 and 7.0. Furthermore, figure 4 indicates that the removal process of MB (adsorption onto TNTs) reached fast equilibrium in approximately 40 min, suggesting that TNTs have a high affinity to MB ions in the solution. However, figure 4 shows that the pH value is higher than the pHpzc value, TNTs can react with base medium leading to the decrease of the TNTs adsorption.
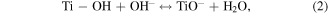
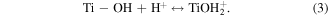
Figure 5 illustrates the effects of contact time and solution pH on MB photocatalytic reaction of hydrothermally synthesised TNTs. To describe the kinetic rate of MB photocatalytic degradation, the obtained experimental data in this study were fitted to the Langmuir-Hinshelwood kinetic equation [40, 41]:
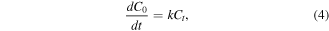
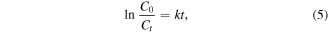
where k (1/min) is the pseudo-first order rate constant, C0 (mg/l) and Ct(mg/l) are defined in equation (1).
Figure 5. MB photodegradation of hydrothermally synthesised TNTs in the medium with pH values from 1 to 6 (a), and from 7 to 12 (b).
Download figure:
Standard image High-resolution imageTable 1 lists the pseudo-first order rate constant determined from the slope of a plot of ln(C0/Ct) versus t. As provided in figure 5(a), the MB photodegradation efficiency of hydrothermally synthesised TNTs increased when pHsolution increased from 1.0 to 6.0, with the optimal pHsolution being obtained at pH 5.0. Similar to photodegradation efficiency, the photodegradation rate (k) also significantly increased within pHsolution from 1.0 to 6.0, and the highest k (7.08 × 10−3 1 min−1) value was found at pHsolution = 5.0 (table 1). Meanwhile, at pHsolution higher than 6.0, TNTs exhibited the fastest photodegradation rate at pH 11 (k = 5.81 × 10−3), but the slowest rate at pH 7 (2.87 × 10−3) (figure 5(b) and table 1). The result suggested that the photodegradation process of MB dye molecules by TNTs was strongly dependent on pHsolution.
Table 1. Pseudo-first order rate constants for photodegradation of MB with TNTs.
| pH | k (10−3 × min) | R2 |
|---|---|---|
| 1 | 1.95 | 0.9916 |
| 2 | 3.65 | 0.9776 |
| 3 | 4.80 | 0.9867 |
| 4 | 5.30 | 0.9983 |
| 5 | 7.08 | 0.9867 |
| 6 | 3.10 | 0.8171 |
| 7 | 2.87 | 0.9083 |
| 8 | 4.31 | 0.8669 |
| 9 | 4.40 | 0.8354 |
| 10 | 3.50 | 0.9813 |
| 11 | 5.81 | 0.9247 |
| 12 | 3.87 | 0.9962 |
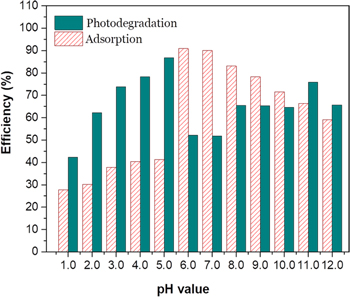
Figure 6 shows the relationship of the MB adsorption and photodegradation by TNTs in the medium with different pH values. This result confirmed that the pH of the medium strongly affected the adsorption capacity and photodegradation efficiency of hydrothermally synthesised TNTs. Generally, the photocatalytic activity of hydrothermally synthesised TNTs increased significantly in pH value from 1 to 5 and decreased in the pH value from 8 to 10. Although the adsorption ability of hydrothermally synthesised TNTs at pH 6 is greatest but their photocatalytic activity is poor. In addition, in the alkaline medium, the photocatalytic efficiency of hydrothermally synthesised TNTs was almost inferior to that of adsorption capacity but in the medium of the solution with pH 11 and 12, the photocatalytic and adsorption abilities do not comply with this rule. The explanation for this result could be proposed that the TNT surface was completely covered by MB solution leading to the photoexcited receiving ability of hydrothermally synthesised TNTs being disadvantage. Therefore, the MB photodegradation of hydrothermally synthesised TNTs in this pH value range is not good. The MB adsorption and photodegradation efficiencies of hydrothermally synthesised TNTs reach the highest values in the medium with pH 6, while the lowest values of the MB adsorption and photodegradation efficiency occur at pH 1. Results of the adsorption and the photocatalytic activity of TNTs are listed in table 2. Therein, the MB adsorption efficiency of hydrothermally synthesised TNTs in medium with pH 1 and pH 6 is 28% and 91%, respectively. Furthermore, the MB photodegradation efficiency of hydrothermally synthesised TNTs after 300 min of the UV irradiation in medium with pH 1 and pH 6 is 42% and 52%, respectively while the highest photocatalytic efficiency (87%) of TNTs is achieved at pH 5 after 300 min under UV irradiation.
Figure 6. Relationship of the MB adsorption and photodegradation of hydrothermally synthesised TNTs after 300 min under UVA irradiation.
Download figure:
Standard image High-resolution imageTable 2. MB adsorption and photocatalytic degradation efficiencies of TNTs.
| pH | Adsorption after 60 min (%) | Photocatalytic degradation after 60 min (%) | Photocatalytic degradation after 300 min (%) |
|---|---|---|---|
| 1 | 28 | 14 | 42 |
| 2 | 30 | 27 | 62 |
| 3 | 38 | 31 | 74 |
| 4 | 40 | 32 | 78 |
| 5 | 41 | 38 | 87 |
| 6 | 91 | 41 | 52 |
| 7 | 90 | 34 | 52 |
| 8 | 83 | 31 | 64.5 |
| 9 | 78 | 42 | 64.4 |
| 10 | 72 | 30 | 64.6 |
| 11 | 45 | 55 | 76 |
| 12 | 60 | 32 | 65.7 |
The MB intensity change for different pH value conditions could be explained by the existence of the H+ or OH− in the medium. These radicals will contribute to the photocatalytic activity of TNTs [5, 42]. When the TNTs are irradiated by UV light, electrons and holes will be generated in the VB of TNTs, and then electrons will transfer to the CB of the TNTs. The existence of the H+ or OH− in the medium will contribute to the photocatalytic activity of TNTs as below equations, therein, the contributions of the H + and OH− are expressed clearly in the equations (2), (4), and (6).
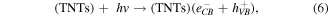
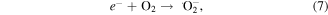
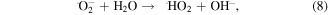
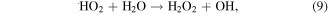
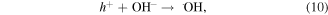
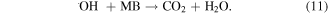
4. Conclusion
This study indicated the effect of the pH value in the solution medium on adsorption and photocatalytic abilities of hydrothermally synthesised TNTs. The adsorption ability of hydrothermally synthesised TNTs is strongest at pH 6 (91%) while the photocatalytic efficiency of hydrothermally synthesised TNTs is strongest at pH 5 with the MB photodegradation efficiency of 87% after 300 min under UV irradiation. The pH values of solution affected the charged state of the TNT surface, which changed the adsorption and photodegradation abilities of the material. This study demonstrated that TNTs can be used to photodegrade dyes at different pH values of the wastewater in the future.
Acknowledgments
This study was done with the support of the CM Thi Laboratory, and Vietnam National University - Ho Chi Minh City (VNU-HCM).