Abstract
In this study the novel approach for fabricating thin film Ag/AgCl electrodes was proposed using electron beam evaporation method for potential measurements as reference electrodes. A silver and silver/silver chloride (Ag/AgCl) thin films with a thickness of 300 nm were evaporated on the silicon substrate which had been initially oxidized to create the insulating silicon dioxide (SiO2) layer. The dimension of the electrodes was about 0.5 mm × 2.0 mm. The characterization of AgCl layers was performed with field emission scanning electron microscopy, x-ray diffraction and energy dispersive x-ray spectroscopy, Raman spectroscopy and optical microscopy. The open circuit potential (OCP) measurements in buffer solution pH 7 were investigated between the fabricated Ag/AgCl electrodes and a commercial Ag/AgCl electrode as the reference electrode. The measured OCP values had insignificant disparity. The good reproducibility and potential stability with the average potential value and deviation were within 334.0 mV ± 4.07 mV. In addition, a pH sensing test was also performed by using a laboratory-made potentiometer, which showed a sensitivity of 48.01 mV pH−1, with the correlation coefficient being greater than 0.99. The results showed that the fabricated thin film Ag/AgCl electrodes which could be obtained by a simple and fast process had high purity and could be used as reference electrodes for potential measurements.
Export citation and abstract BibTeX RIS
1. Introduction
In recent years micro-fabrication techniques have been used for fabricating portable electrochemical sensors due to their advantages such as simplicity process, high throughput and low cost [1]. However, conventional Ag/AgCl electrodes are normally in macro-scale which cannot be employed in micro-electrochemical sensors [2]. Thus, many scientists are trying to minimize and integrate the reference electrode with the other sensors on a chip for biomedical and environmental applications [3, 4]. In the early years of this century, the studies on miniaturization of Ag/AgCl reference electrodes have been published rapidly.
The two main components of the electrochemical sensor system are the reference electrode (RE) and the working electrode (WE). The reference electrode is a crucial component in electrochemical measurements [5]. The important function of a reference electrode is to provide constant potential during electrochemical measurement. Standard hydrogen electrode (SHE), saturated calomel (Hg/Hg2Cl2) electrode (SCE) or silver/silver chloride (Ag/AgCl) in saturated KCl solution are the most common reference electrodes which are widely used many applications. The reason that made those RE becomes so popular is due to the 'wet chemistry' in their construction in the form of the electrolyte solution. Depending on the purpose of the experiment and the budget of the project, the scientist can choose the reference electrode which is the most suitable for them [6]. One of the main problem of these common reference electrodes is that they have large size and complicated structure which will restrict the further application of these electrodes in order to build a compact sensors [7]. The Ag/AgCl reference electrode is applied in the potentiometric sensors due to its convenient properties such as stability, easy maintenance, miniaturization and non-toxicity [8, 9]. In addition, the Ag/AgCl electrode is a widely used reference electrode because it is simple, inexpensive, very stable and non-toxic. In order to fabricating the Ag/AgCl electrode, one of the main crucial issues is fabrication of the thin layer of Ag and AgCl. Micro-fabrication techniques are the efficient solution for this issue, that lead to the widely evolve of this technique [10–15]. There are many ways to fabricate micro-electrodes, such as electrochemical deposition [14, 16], screen printing [6, 17, 18], physical and chemical vapour deposition (sputtering, e-beam etc) [12, 17]. In the late 19th century, one of the solutions used a combination between electro-deposition (using the sputtering method to deposite Ag thin film on the surface of the electrode) and electrochemical (using chemical method is combine 1 M FeCl3 and 1 mM 6-mercepto-1-hexanol (MCH) to create the AgCl thin film on the top of the Ag layer) to fabricate Ag/AgCl electrodes [19]. The screen printing method, which can be an alternative method, is simple and has low cost but it is not reproducible. On the other hand, the electro-deposited Ag layer and sputtered Ag layer can be converted to AgCl via an anodic oxidation and chlorination [20–22].
In this study the thin film Ag/AgCl electrodes were simply fabricated via the new method by using the electron beam evaporation techniques (e-beam) on the silicon/silicon dioxide (Si/SiO2) substrate. A miniaturized reference electrode was formed as a reference for potential measurements.
2. Experimental
2.1. Materials
Silver nitrate (AgNO3) 99% was purchased from Scharlab S L, Spain. Sulfuric acid (H2SO4) 98%, hydrogen peroxide (H2O2) 30%, sodium chloride (NaCl) were obtained from Merck, Germany. All solutions were prepared in deionized water that was supplied by a DI system (Purelab Ultra, Elga Co., UK), the resistance of the outlet water was 18.2 MΩ. Standard buffer solutions of different pH values were prepared with appropriate mixtures of citric acid (C6H8O7.H2O) and boric acid (H3BO3) obtained from Merck (Germany), tri-sodium orthophosphate (Na3PO4.12H2O) obtained from Sigma–Aldrich (Germany), the pH 7 buffer solution from Hanna instruments (Romania) was used as electrolyte in the potential measurements. Acetone, isopropanol and ethanol were purchased from Merck (Germany). All chemicals were analytical grade and used with high purity (more than 99%). 4' wafer silicons (p-type, thickness of 380 μm, resistivity of 5–10 Ω cm) which were purchased from Okmetic (Finland) was oxidized to create the insulating layer (SiO2) by the oxidation furnace PEO 601 (ATV, Germany).
2.2. Fabrication of the thin film Ag/AgCl electrodes
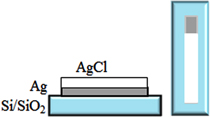
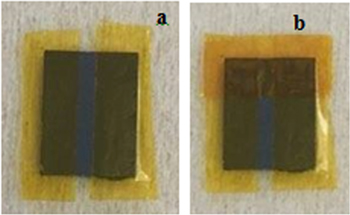
e–beam evaporation method was used to fabricate the reference electrodes. At first, the Si/SiO2 substrate was cleaned with a piranha solution (mixture of H2SO4:H2O2 with ratio of 3:1), then under ultrasonic about 5 min in acetone, washed again with ethanol, isopropanol, DI water and dried by blowing nitrogen gas. The polyimide sticking plasters were used as the masks to create the shape of the electrodes (figure 1). A cross-sectional view of the Ag/AgCl reference electrode is shown in figure 1. Then, silver was evaporated on the Si/SiO2 substrate by using an e–beam evaporator (Torr International Inc., USA). The sticking plasters were used to cover a part of silver coated layer (figure 2).
Figure 1. The design and structural cross-section of the Ag/AgCl micro-electrode on the Si/SiO2 wafer.
Download figure:
Standard image High-resolution imageFigure 2. The images of the electrode on the Si/SiO2 substrate by using sticking plaster to evaporated (a) Ag, and (b) AgCl.
Download figure:
Standard image High-resolution imageAfter that, silver chloride (AgCl) was fabricated on the Si/SiO2/Ag substrate. AgCl was achieved by mixing 1 ml silver nitrate (AgNO3) 0.2 M and 1 ml sodium chloride (NaCl). The balanced chemical equation described above is written as follows
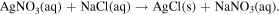
The precipitate was filtered and then washed 8 times with DI water in order to eliminate the residual reactants and dried at 80 °C for 30 min in the vacuum oven. Finally, the dried AgCl powder was used as the e-beam target in order to fabricate the AgCl thin film on the Ag layer. The silver and silver chloride layers were fabricated with parameters shown in the table 1.
Table 1. The parameters of the fabricated Ag/AgCl electrode by e-beam evaporator.
| Material | P (mbar) | U (kV) | I (mA) | Rate (Å/s) |
|---|---|---|---|---|
| Ag | 9.10−6 | 4.95 | 25–30 | 1000 |
| AgCl | 9.10−6 | 4.95 | 5–8 | 1000 |
2.3. Characterization and measurements
2.3.1. Characterization
The thickness of the Ag/AgCl electrodes was measured by Stylus Profiler Dektak 6 M (Veeco, USA), the surface morphology was observed with field emission scanning electron microscopy FESEM (SU8000, Hitachi, Japan). The composition of Ag/AgCl film was detected by x-ray diffraction spectrometer (XRD) using diffractometer D500 (Siemens, Germany), energy dispersive x-ray spectroscopy (EDS), micro Raman spectroscopy with Lab RAM 300 (Jobin Yvon, France).
2.3.2. Potential measurements
An electrochemical workstation (Autolab PGSTST—302 N, Methohm, the Netherlands) was used to measure the open circuit potential (OCP) in the pH buffer solutions. During the OCP measurements, the fabricated Ag/AgCl electrodes and the commercial Ag/AgCl reference electrode in saturated KCl electrolyte solution (Basi Inc., US) were used alternately as the reference electrode of the electrochemical system to compare their performances. A thin film Pt (width of 0.5 mm × length of 1.5 mm) was used as a working electrode.
3. Results and discussion
3.1. Characterization of the fabricated Ag/AgCl electrodes
Figure 3 shows the image of the thin film Ag/AgCl electrode after being fabricated and the thickness of the Ag and AgCl layers was measured by Stylus Profiler (Dektak 6 M, Veeco, USA). The silver layer had thickness of 105 ± 10 nm and the thickness of the silver chloride layers was 250 ± 10 nm.
Figure 3. The photograph of the fabricated Ag/AgCl after being evaporated by e–beam evaporator.
Download figure:
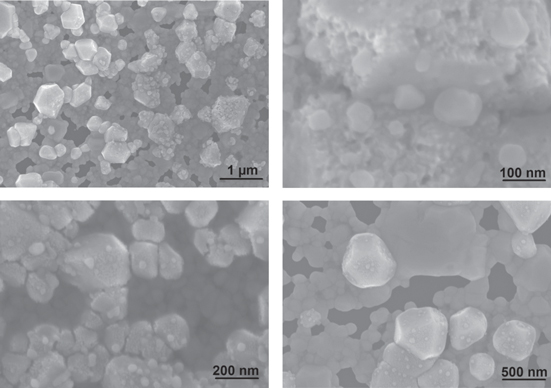
Standard image High-resolution imageThe fabricated Ag/AgCl electrodes were studied by using FESEM to observe the changes in their surface morphologies (figure 4). From figure 4, it clearly shows that the surface morphologies of the AgCl layers were granular with pores. The diameters of the grains were divergent because the amperage was not stable during the evaporation process (table 1). On the surface of the electrode, the AgCl granules were interconnected more tightly. A great variety of AgCl grain size ranging from less than 100 nm to 250 nm was obtained.
Figure 4. The FESEM images of the fabricated Ag/AgCl electrodes in different magnifications.
Download figure:
Standard image High-resolution imageChemical analysis of the Ag/AgCl layers fabricated on the Si/SiO2 substrates was performed by using energy dispersive x-ray spectroscopy (EDS) with normal scan and mapping modes. In figure 5 it clearly shows that the AgCl layer was successfully fabricated on the surface of the SiO2 layer with the appearance of the Ag, Cl, Si and O peak elements, the molar ratio of Ag:Cl is 3:1. The results showed that the Ag/AgCl electrodes were produced with high purity.
Figure 5. The EDS spectrum of the Ag/AgCl layer (a) and EDS mapping of Ag (b), Cl (c), Si (d), and O (e).
Download figure:
Standard image High-resolution imageMoreover, the bonding between Ag+ and Cl− was determined by using the x-ray diffraction (XRD) method. Also, from the XRD result, the lattice system and the lattice constant of the AgCl crystal could be calculated. The XRD result was shown in figure 6. The XRD scan was run from the 20° to 80°. From XRD patterns, it was clearly seen that the AgCl crystal which was fabricated in this article had the face centre cubic (fcc) structure. The crystallinity of the AgCl nanoparticles revealed the existence of major 6 peaks at 2θ value of 27.91°, 32.32°, 46.27°, 55.01°, 57.50° and 68.69°. This completely fitted the result from the Joint Committee on Powder Diffraction Standards (JCPDS file 31-1238). The AgCl thin film had the major miller plane of (111).
Figure 6. XRD spectrum of the fabricated thin film AgCl.
Download figure:
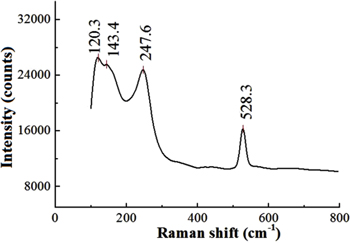
Standard image High-resolution imageSilver chloride is an ionic metal—halide compound, with a fcc lattice structure in which each Ag+ is surrounded by an octahedron of six chloride ligands. AgCl exhibit two bands related to the stretching vibration of the bridging metal—halogen (Ag–Cl). To further investigate the coated Ag/AgCl electrode structure, Raman spectroscopy was carried out. Figure 7 shows that the Raman spectrum of Ag–Cl bond to the Si/SiO2 substrate could be found in the Raman shift at 120.3, 143.4, 247.6 cm−1 corresponding to 95, 143, 233 cm−1 of the standard Raman spectrum [23] and 528.3 cm−1 of the silicon Raman peak [24].
Figure 7. The Raman spectrum of the fabricated Ag/AgCl electrode.
Download figure:
Standard image High-resolution image3.2. Potential measurements
3.2.1. Reproducibility of the electrodes and the stability of the fabrication technology
The potential and response time of the fabricated Ag/AgCl electrodes were measured in buffer solution pH 7 versus the commercial Ag/AgCl reference electrode. The fabricated Ag/AgCl electrodes quickly reached the equilibrium within 10 s after the electrodes were immersed in the solutions during each measurement (figure 8). Figure 8(b) shows that the good reproducibility and potential stability with potential value and deviation was within 334 ± 1 mV. This divergence was due to the fabrication process, the different shapes and size of the Ag/AgCl reference electrode.
Figure 8. The response times of a single fabricated Ag/AgCl electrode (a) and the reproducibility, stability of 4 different fabricated Ag/AgCl electrodes (b) were measured in pH 7 solution.
Download figure:
Standard image High-resolution image3.2.2. Effect of pH on the potential of electrode
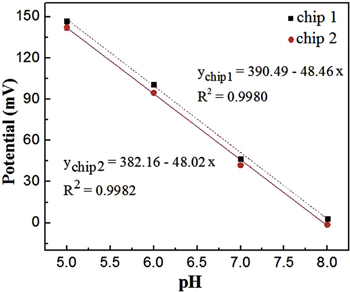
The sensitivity of the pH sensor was investigated in solution with pH levels from 5 to 8. The electrode was immersed into the electrolyte solutions. The potentials were recorded when the fabricated Ag/AgCl electrode was used as the reference electrode and the platinum electrode was used as the working electrode. The potential response was plotted (figure 9). The fabricated electrodes exhibited a linear correlation in measured pH ranges and the sensitivity was calculated to be –48 mV pH−1 and the linear correlation coefficient was larger than 0.99 over the pH levels of 5–8. These results agreed very well with theoretical value of 59.2 mV pH−1 [25].
Figure 9. The linear relationship of the potential and pH of the electrodes from pH 5 to 8.
Download figure:
Standard image High-resolution image4. Conclusions
In this paper, the thin film Ag/AgCl electrodes were designed, fabricated and characterized via e–beam evaporation method. The electrodes had size of approximately 0.5 mm × 2.0 mm and a thin thickness of 350 ± 10 nm with the high purity. The electrodes exhibited excellent performance with fast response and steadiness. The fabricated Ag/AgCl electrode showed a good reproducibility and potential stability of 334 ± 1 mV. The pH sensor had a sensitivity slope is −48.01 mV pH−1 (R2 > 0.99) and had a fast responding time (10 s). The results showed that the thin film Ag/AgCl electrodes could be integrated on potentiometric sensors as a commercial reference electrode. In addition, the fabricated Ag/AgCl electrodes could be miniaturized and then coated with a protection layer for the further use in the water analysis by using electrochemical technique.
Acknowledgments
The research group highly appreciate the kind funding support of Ministry of Science and Technology for this research under the grant number 15/2017/ĐTĐL.CN-XHTN.