Abstract
This paper presents an overview of syntheses and applications of magnetic nanoparticles (MNPs) at the Institute of Materials Science, Vietnam Academy of Science and Technology. Three families of oxide MNPs, magnetite, manganite and spinel ferrite materials, were prepared in various ways: coprecipitation, sol–gel and high energy mechanical milling. Basic properties of MNPs were characterized by Vibrating Sample Magnetometer (VSM) and Physical Properties Measurement Systems (PPMS). As for biomedical application, the aim was to design a novel multifunctional, nanosized magnetofluorescent water-dispersible Fe3O4-curcumin conjugate, and its ability to label, target and treat tumor cells was described. The conjugate possesses a magnetic nano Fe3O4 core, chitosan (CS) or Oleic acid (OL) as an outer shell and entrapped curcumin (Cur), serving the dual function of naturally autofluorescent dye as well as antitumor model drug. Fe3O4-Cur conjugate exhibited a high loading cellular uptake with the help of a macrophage, which was clearly visualized dually by Fluorescence Microscope and Laser Scanning Confocal Microscope (LSCM), as well as by magnetization measurement (PPMS). A preliminary magnetic resonance imaging (MRI) study also showed a clear contrast enhancement by using the conjugate. As for the environmental aspect, the use of magnetite MNPs for the removal of heavy toxic metals, such as Arsenic (As) and Lead (Pb), from contaminated water was studied.
Export citation and abstract BibTeX RIS

Content from this work may be used under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Magnetic nanoparticles (MNPs) are attractive to many researchers because of their wide-ranging applications of data storage, magnetic fluids, catalysis, biotechnology, biomedicine and environmental remediation [1–4]. Several methods have been developed for synthesizing MNPs with different compositions. MNPs with appropriate modified surfaces have been widely applied in biomedical applications, such as diagnostic (magnetic resonance imaging and magnetic enhanced enzyme-linked immunoassay) and therapeutic (drug delivery and hyperthermia) applications. The magnetites have been studied for hyperthermia and considered as the novel environmental treatment [5]. The adsorbability of MNPs, applying for toxic metals (Arsenic and Lead) in contaminated water, has also been demonstrated. However, naked MNPs still have disadvantages. It is most important to stabilize the MNPs, preventing agglomeration, which reduces the surface area of the materials, and to keep naked MNPs from being oxidized, which destroys their magnetism. Thus, it is crucial to chemically stabilize the morphology and magnetism of MNPs, during and after tsynthesis with non-toxic biocompatible protecting layers [6–8].
In our studies, chitosan (CS), an excellent biocompatible and biodegradable polymer with a high content of amino groups (–NH 2), making it possible to form metal complexes to enhance the surface for drug delivery; oleic acid (OL), a common natural substance that is nontoxic and able to treat chronic diseases; and curcumin (Cur), which supplies us with a multi-functional method of fluorescent and magnetic imaging as well as a cancer treatment, were chosen. Our aims were (i) to study the magnetic heating effect (MH) of different MNPs and (ii) to fabricate CS- or OL-coating Fe 3 O 4-Cur conjugates with a diameter of <500 nm using macrophages as vehicles to carry them to the tumors.
2. Experimental
2.1. Synthesis of magnetic nanoparticles
All iron oxide nanoparticles were prepared by using the co-precipitation process. In brief, iron oxide nanoparticles were synthesized from iron chloride solutions (with Fe 3+/Fe 2+ ratio of 2 : 1) with the presence of NH 4 OH or NaOH. Most of the manganite nanoparticles of La 0.7 Sr 0.15 Ca 0.15 MnO 3 were prepared by using the conventional solid-state reaction method followed by a milling process and a secondary annealing treatment using La 2 O 3, SrCO 3, CaCO 3 and MnO 2 powders as starting materials (SC900), while nanoparticles of Mn 1−x Zn x Fe 2 O 4 ferrite (x=0.0–0.5) were synthesized by using the co-precipitation method with MnCl 2, FeCl 3 and ZnCl 2 as the starting materials and NaOH as the precipitating agent (Z 10).
2.2. Fe3 O4-Cur conjugate preparation
CS coated Fe 3 O 4 fluid (CSF) was prepared by chemical co-precipitation of Fe 2+ and Fe 3+ ions by NaOH in the presence of CS, according to the detailed procedure described in [9]. OL coated Fe 3 O 4 fluid (OLF) was prepared by multistep synthesis [10]. Briefly, OLF and CSF were synthesized by co-precipitation from iron chloride solution with an Fe 3+/Fe 2+ ratio of 2 : 1. Then, Curcumin (Cur, preliminarily solubilized in ethanol) was attached by adsorption on the Fe 3 O 4 surface of OLF/CSF. Thus, several types of ferrofluids without/with Cur were prepared for further fluorescent and magnetic imaging.
2.3. Structural and magnetization characterization
Ultraviolet-Visible (UV-Vis) spectra were recorded by a UV-Vis Agilent 8453 spectrophotometer in the range of 250–800 nm. Laser Scanning Confocal Microscope (LSCM) images with excitation light of 488 nm were collected using a ZEISS 510 LSCM with a 40× or 63× oil immersion objective. Magnetic properties were characterized by using a home-made Vibrating Sample Magnetometer (VSM) and a Physical Property Measurement System (PPMS, Quantum Design) at fields ranging from −20 to 20 kOe at 25 °C, with an accuracy of 10−5 emu. The images of a mice tumor were carried out by a Philips Intera 1.5 Tesla MR scanner (Netherlands) with a slice thickness of 3 mm on transversal and coronal planes, and using two sequences—T2-weighted and T1-weighted.
2.4. Magnetic heating experiment
A commercial generator (RDO HFI 5 kW) was used to create an alternating magnetic field of amplitude from 40 Oe to 100 Oe at 219 kHz (figure 1). The induction coil has seven turns with 3 cm diameter and 11.5 cm length. Powder samples of weighed mass were dispersed in 0.5 ml of water and kept in a round-bottomed glass holder. A vacuum layer was used to insulate thermal exchange from the samples to the ambient medium. Temperature increases in the range from 0 °C to 100 °C and from 0 °C to 200 °C were measured by using an alcoholic thermometer and a Copper–Constantan thermocouple, respectively. The magnetic heating effect was thoroughly studied for several naked particles samples of all oxide materials and for the starch-coated magnetite nanosystem. The measured time period was from 20 to 30 min. The saturation temperature, T s , was defined as the one gained at a heating time of 25 min.
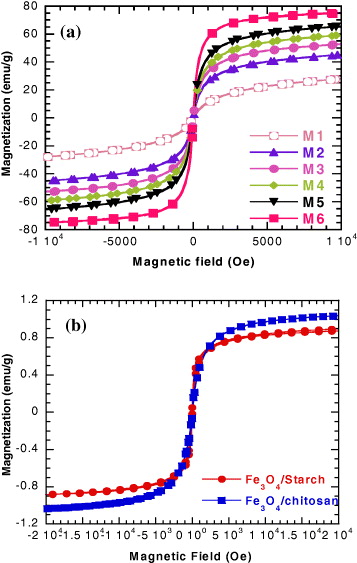
Figure 1 Magnetization curves for Mi samples of naked magnetite NPs (a) and the polymer/magnetite ferrofluids (b).
2.5. Adsorption/desorption experiments
2.5.1. Adsorption of As on magnetite nanoparticles
A stoke solution of arsenic-contaminated water with an arsenic concentration of 500 μg l −1 was prepared and used in all experiments. Firstly, iron oxide nanoparticles were dispersed in the stoke solution for 30 min using an ultrasonic bath. After that, the solution was passed through a cylindrical column containing some steel foils under the strong magnetic field of a permanent magnet (not shown). The water after separation was collected and capped into a glass bottle. The arsenic concentration of this water was then analyzed by using high resolution absorption spectrometry.
2.5.2. Adsorption of Pb(II) on chitosan/magnetite composite
Chitosan/magnetite nanocomposite beads were used to adsorb Pb(II) from aqueous solutions at pH 4–6 at room temperature with a concentration of bead to water of 100 mg per liter of water. The adsorption of the heavy metal ions was investigated in the range of 1060 mg l −1 for the Pb(II). The concentrations of this ion were analyzed by a UV-Vis method.
3. Results and discussion
3.1. Basic characteristics of synthesized nanoparticles
Table 1 presents characteristic parameters: magnetization at 1 Tesla (M1T ), coercivity (H c ), Curie temperature (T C ), particle diameter (D), specific loss power (SLP), and saturation temperature (T s ) of the three samples of mangetite (M 6), manganite (SC 900) and spinel ferrite (Z 10) nanoparticles.
Table 1. Basic magnetic properties and heating parameters of three MNP samples.
| M6 | 75 | 10 | 20 | 107 | ||
| SC900 | 46 | 61 | 53 | 24 | 49 | |
| Z10 | 62 | 375 | 2 | 130 |
Figure 1 shows magnetization curves of magnetite NPs (figure 1(a)) and the two ferrofluids of polymer/magnetic NPs (figure 1(b)). All of the curves' behaviors indicate that the magnetic nanoparticles are of superparamagnetic type. The saturation magnetizations of the Fe 3 O 4/ST and Fe 3 O 4/CS are, respectively, of about 0.9 and 1.1 emu g −1, which give calculated concentrations of magnetite NPs equal to 15 and 20 mg ml −1, correspondingly.
3.2. Magnetic heating of the MNPs
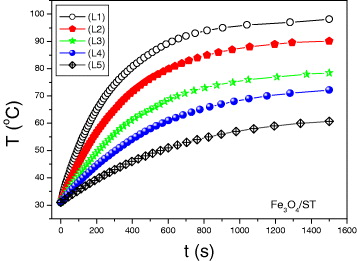
Figure 2 shows MH curves measured for the starch-coated Fe 3 O 4 magnetic sample with various Fe 3 O 4/ST concentrations.
Figure 2 Magnetic heating curves measured for Fe 3 O 4/ST ferrofluid at various concentrations.
3.3. Removal of arsenic and lead
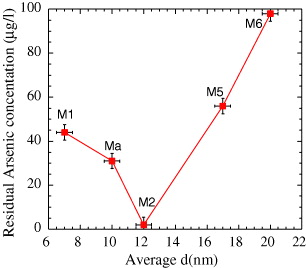
The dependence of the residual arsenic concentration in treated water on the size D of magnetite NPs is presented in figure 3. With particle diameter D decreasing from 20 to 7 nm, the residual arsenic concentration first decreases very strongly to get a minimum value (1.6 μg l −1) at D∼12 nm, and then increases with a further decrease of D.
Figure 3 As filtration capability as a function of the diameter of Fe 3 O 4 MNPs of the Mi series.
The decrease in the residual As in the range of D=20–12 nm can be explained by the increase in particle adsorption due to the increase in surface area. With further decreasing D, although the adsorption of As continuously increases, the magnetic force starts to fail to overcome the Brownian motion and viscous drag in the MNPs–As ferrofluid.
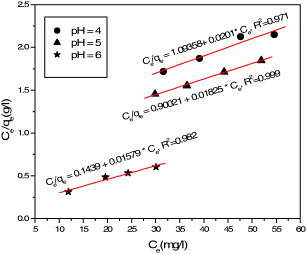
Figure 4 shows a plot of C e /q versus C e for the case of adsorption of Pb(II) ions for pH=4, 5 and 6, from which the linearity fit gave the q m values, respectively, of 49.55, 54.80 and 6.33 mg g −1 (q m is the maximum adsorption of metal ions). These results permitted us to conclude that magnetite/chitosan nanocomposite beads could serve as a promising adsorbent for heavy metals.
Figure 4 Langmuir isotherm of the Pb(II) adsorption on chitosan/magnetite composite.
3.4. Biomedical application
It is well known that surface coatings provide a steric barrier to prevent nanoparticle agglomeration and avoid opsonization (the uptake by the reticuloendothelial system (RES), thus shortening circulation time in the blood and MNPs' ability to target the drug to specific sites and reduce side effects). In addition, these coatings provide a means to tailor the surface properties of MNPs, such as surface charge and chemical functionality. Some critical aspects with regard to polymeric coatings that may affect the performance of an MNP system include the nature of the chemical structure of the polymer (e.g. hydrophilicity/hydrophobicity, biodegradation), its molecular weight and conformation, the manner in which the polymer is anchored or attached (e.g. electrostatic, covalent bonding) and the degree of particle surface coverage. A variety of natural polymers/surfactants have been evaluated for this purpose. The most widely utilized and successful coatings, in terms of in vivo applications, are dextran, PEG, chitosan (CS) and oleic acid (OL).
By conjugating Fe 3 O 4 with Cur, it can be logically expected that the conjugate can be used as a cancer drug and the drug uptake can be observed in situ by fluorescence as well as magnetic measurements. On the UV-Vis spectra (not shown), the Cur containing fluids exhibited an absorption band at a wavelength of around 425 nm. This UV absorption effect explains the green fluorescence observed under fluorescence microscope, as demonstrated in an example image measured for the OLF-Cur sample excited by 488 nm Argon laser.
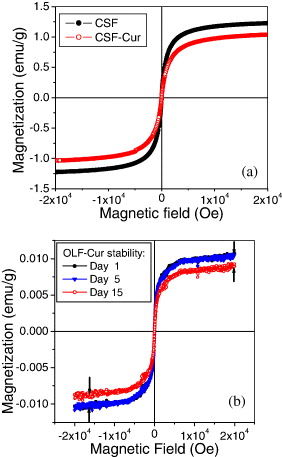
Figure 5 presents the M(H) curves taken for CSF and CSF-Cur. On the basis of the saturation magnetization value of the non-coated Fe 3 O 4 NPs (M s =70 emu g −1), the Fe 3 O 4 concentration can be estimated as 17.5 and 14.7 mg ml −1 for CSF and CSF-Cur, respectively. It is worth noting that CSF and OLF are magnetically stable in distilled water for several weeks. However, in a physiological solution (e.g. 1×PBS, pH=7.4), the stability of CSF and CSF-Cur deteriorated drastically to a few hours, whereas the OLF and OLF-Cur still maintained their remarkable stability, at least for 5–7 days.
Figure 5 Magnetization versus field for CSF and CSF-Cur (a) and OLF-Cur diluted in PBS measured at various maintenance times (b) [11].
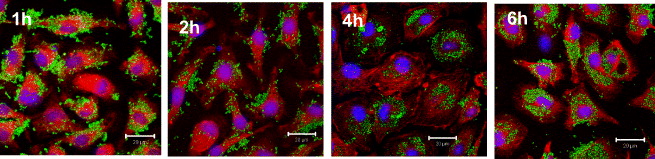
Further, to get a closer insight into the kinetics, Fe 3 O 4-Cur uptake was visualized by LCSM in situ images, taken at 1, 2, 4 and 6 h of incubation. As expected, the number of Fe 3 O 4-Cur taken up into macrophage cytoplasm increases clearly with incubation time. The green fluorescent color is noticeably seen surrounding the nucleus surface at 0.5–1 h, then appears increasingly inside the nucleus at 2–4 h and finally reaches its maximal intensity there at 6 h. Since the fluorescence intensity of Cur directly correlates to the internalization ability of Fe 3 O 4-Cur into cells, it can be concluded that the Fe 3 O 4-Cur particles are efficiently internalized (figure 6).
Figure 6 Uptake kinetic observed in situ by LSCM (image taken at 1, 2, 4 and 6 h of phagocytosis of OLF-Cur).
To investigate whether these nanocarriers of CSF and OLF could be used advantageously for magnetic resonance imaging, tumor-bearing mouse were prepared by intra-peritoneal injection of thiopental. When tumors reached a size of about 8×11 mm, the nanoparticles (OLF-Cur) were introduced to the tumors by intra-tumor injection directly. A healthy mouse and a tumor-bearing mouse injected with the equivalent volume of PBS were used as controls. The mice were then imaged by the Philips Intera 1.5 Tesla MR scanner with a slice thickness of 3 mm on transversal using T2-weighted sequences. Each scanning took about 5–7 min. While there was almost no significant difference in the tumor's signal intensity as compared with the control, the intra-tumor injection of OLF-Cur resulted in reducing the MR signal intensity, which in turn made the invaded region black. Thanks to this contrast change, the tumor could easily be differentiated from the surrounding tissues.
4. Conclusion
We were successful in synthesizing MNPs by using different methods, such as co-precipitation, sol–gel and high energy mechanical milling. The as-synthesized conjugates of magnetite and curcumin (coated by chitosan or oleic acid) had good fluorescent and magnetic tracing ability. The dual-tracing method clearly helped us to interpret and measure data in testing phagocytosis. The MNPs also exhibited excellent bioadsorbability with respect to toxins and heavy metals, like arsenic and lead. The adsorption and desorption were fully investigated, in which the diameters of MNPs ranging from 7–15 nm was the optimal value for the most efficient adsorption and desorption of those metals. The hyperthermia process was also open to the new and practical approach of regenerating sorbent as well as manipulating conjugates for further biomedical applications.
Acknowledgments
This work was performed under the financial support of a Ministry of Science and Technology application-oriented grant, a Vietnam Academy of Science and Technology grant, and a Korean-Vietnam grant.