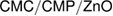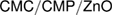Abstract
New polymeric system containing two of carbohydrate based polymers, namely, carboxymethyl chitosan (CMC) and carboxymethyl pullulan (CMP) have been prepared via dispersion polymerization. Poly (N-isopropylacrylamide) (PNIPAAm) have been used in the polymerisation reaction as a temperature sensitive polymer and silane based crosslinker have been used for crosslinking reaction between the selected polymers. The prepared composite was characterized using Fourier transform infrared spectroscopy (FT-IR), scanning electron microscopy (SEM), swelling measurements and responsibility to both temperature and pH changes. Moreover, zinc oxide have been synthesised and characterized using transmission electron microscopy (TEM). The as prepared nanoparticles have been added to the hydrogel network to increase the antibacterial activity of both synthesised gel and the treated fabrics. The prepared composite have been incorporated to cellulosic material using pad dry-cure technique using a cross-linking agent. Stimuli responsiveness properties of cellulosic materials have been confirmed by measuring the effect of pH and temperature on the water uptake.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 3.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Hydrogels are characterized as three-dimensional systems of hydrophilic polymers, which can adsorb water and swell; however, they are not dissolved. Moreover, it is clear that these hydrogels could react to ambient environment and simultaneously demonstrate their changes in both chemical and physical properties [1, 2]. Hydrogels having the pH and/or temperature-sensitive properties have been in focus because of their extraordinary potential for bioengineering and biomedical utilize, to be specific, drug release, molecular separation, cell culture, etc [3, 4].
There are many polymers which can be utilized as temperature sensitive polymers, one of them is Poly(N-isopropylacrylamide) (PNIPAAm). This polymer is one of the best polymers used for producing thermo-sensitive textile, because it has a sharp phase point (lower critical solution temperature; LCST), which is closed to temperature of human body and it is about  [5, 6].
[5, 6].
Due to their properties such as biocompatibility, renewability and biodegradability, polysaccharides have picked up an essential part on the improving of novel naturally and eco-friendly coatings and bundling materials [7–12]. However, in nature, most polysaccharides are not biocides [13]. Therefore, antimicrobial activity has been accomplished by including inorganic or natural biocide materials to the biopolymers, for example, metal nanoparticles (Ag,  and
and  nanoparticles) [13–20], or by introducing bactericidal moieties to the polysaccharides backbone [21], such as quaternary ammonium groups.
nanoparticles) [13–20], or by introducing bactericidal moieties to the polysaccharides backbone [21], such as quaternary ammonium groups.
One of the important linear polysaccharides is pullulan. It is consist of a repeated glucose unit linked as a ( ) [22, 23]. Pullulan has excellent physical properties, such as, water solubility, adhesive properties. Further properties for pullulan are anti-inflammatory, antithrombotic and anticoagulant properties. These properties made the pullulan easily to utilise as flocculent, foaming, adhesive agent [24, 25]. In addition, composites having pullulan inside their structure shows good antibacterial properties [26].
) [22, 23]. Pullulan has excellent physical properties, such as, water solubility, adhesive properties. Further properties for pullulan are anti-inflammatory, antithrombotic and anticoagulant properties. These properties made the pullulan easily to utilise as flocculent, foaming, adhesive agent [24, 25]. In addition, composites having pullulan inside their structure shows good antibacterial properties [26].
In the present work, an antimicrobial gel containing carboxymethyl pullulan, carboxymethyl chitosan and silane based cross linker have been synthesized by polymerization of biopolymers derivatives, N-isopropylacrylamide and active silane based compound. The functionalization of pullulan was confirmed by infrared and the synthesised gel has been characterized towards pH and temperature effects, mechanical properties and antimicrobial activity, have been evaluated. The produced gel has been used as functional finishing agent for cotton fabrics.
2. Experimental
2.1. Materials
Pullulan (P) was purchased from natural Lab Ltd (USA). Chitosan medium molecular weight (Fluka), Zinc nitrate hexahydrate ( ), N-isopropylacrylamide (NIPAAm) and (3-Glycidyloxypropyl)trimethoxysilane (GPTMS) and hexamethylenetetramine (HMT) (Sigma-Aldrich, Germany). Ammonium persulfate (APS), sodium hydroxide (
), N-isopropylacrylamide (NIPAAm) and (3-Glycidyloxypropyl)trimethoxysilane (GPTMS) and hexamethylenetetramine (HMT) (Sigma-Aldrich, Germany). Ammonium persulfate (APS), sodium hydroxide ( ) and Glutaraldehyde (GA),
) and Glutaraldehyde (GA),  tetramethylethylenediamine (TEMED) and
tetramethylethylenediamine (TEMED) and  methylenebisacrylamide (BIS) were purchase from Fluka, Germany, monochloroacetic acid (MCA) was purchase from Merck, Germany.
methylenebisacrylamide (BIS) were purchase from Fluka, Germany, monochloroacetic acid (MCA) was purchase from Merck, Germany.
2.2. Preparation
2.2.1. Preparation of cross-linked O-carboxymethyl chitosan.
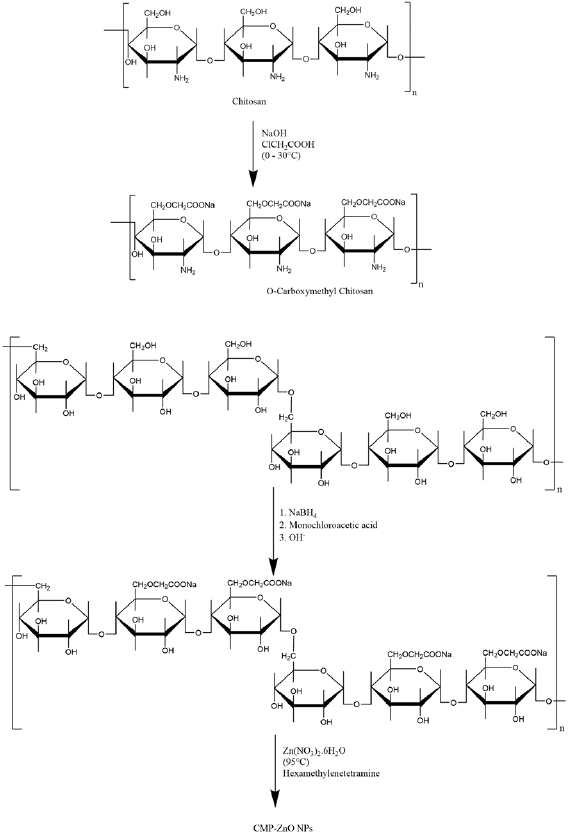
O-carboxymethyl chitosan was prepared as illustrated in scheme 1 [27] it can describe in brief as: 3 g of chitosan was dissolved in 80 ml isopropanol (0.2 M). Then 25 ml 
 was added dropwise during 30 min and then 3.5 g of MCA in 20 ml isopropanol was added drop by drop to the above mixture in a 250 ml round flask. The final mixture was stirred for 24 h at room temperature. After that, excess
was added dropwise during 30 min and then 3.5 g of MCA in 20 ml isopropanol was added drop by drop to the above mixture in a 250 ml round flask. The final mixture was stirred for 24 h at room temperature. After that, excess  was added to precipitate O-carboxymethyl chitosan, the precipitate was washed with ethanol until the pH reached 7. The finally obtained material (O-CMC) was dried in vacuum [28].
was added to precipitate O-carboxymethyl chitosan, the precipitate was washed with ethanol until the pH reached 7. The finally obtained material (O-CMC) was dried in vacuum [28].
Scheme 1. Scheme for synthesis of O-CMC and CMP-ZnO nanoparticles.
Download figure:
Standard image High-resolution image2.2.2. Synthesis of carboxymethyl pullulan (CMP).
Carboxymethyl pullulan have been prepared according to Asmarandei et al method [29] as follows: in a 250 round flask, pullulan (10 g),  (0.03 g) have been mixed in 20 ml of water. Then,
(0.03 g) have been mixed in 20 ml of water. Then,  (20 ml, 38 % w/v) and MCA (17.5 g) were added sequentially in three steps. At the first one 10 ml of
(20 ml, 38 % w/v) and MCA (17.5 g) were added sequentially in three steps. At the first one 10 ml of  and 9 g of MCA were added and stirred at
and 9 g of MCA were added and stirred at  for 1 h. Secondly, 5 ml of
for 1 h. Secondly, 5 ml of  and 4.5 g of MCA were added and stirred at
and 4.5 g of MCA were added and stirred at  for 1 h and the stirring continued for additionally 30 min at the same temperature. Lastly step, 5 ml of
for 1 h and the stirring continued for additionally 30 min at the same temperature. Lastly step, 5 ml of  and 4.5 g of MCA were added and stirred at
and 4.5 g of MCA were added and stirred at  for 18 h. Obtained material was dialyzed against distilled water to remove the chlorine ions (detecting with
for 18 h. Obtained material was dialyzed against distilled water to remove the chlorine ions (detecting with  solution). Finally, CMP (as illustrated in scheme 1) was recovered through lyophilisation [30].
solution). Finally, CMP (as illustrated in scheme 1) was recovered through lyophilisation [30].
2.2.3. Synthesis of ZnO nanoparticles (NPs).
Zinc nitrate hexahydrate solution (0.1 M) and hexamethylenetetramine (HMT) have been mixed with solution of prepared carboxymethyl pullulan  at room temperature, then, temperature has been raised to
at room temperature, then, temperature has been raised to  with continues stirring for additional 90 min to obtain zinc oxide suspension. The suspensions was cooled to room temperature and separated through
with continues stirring for additional 90 min to obtain zinc oxide suspension. The suspensions was cooled to room temperature and separated through  membrane. Final precipitate was washed with deionized water and dried for 2 d in vacuum at
membrane. Final precipitate was washed with deionized water and dried for 2 d in vacuum at  .
.
2.2.4. Preparation of the CMC/CMP/ZnO nanogels.
 nanogels have been prepared prepared as illustrated in scheme 2 in three steps as follow: (a) 2 g of O-CMC has been dissolved in 100 ml distilled water. The solution has been stirred at room temperature overnight. GPTMS has been added with half ratio of O-CMC to the solutions under vigorous stirring at
nanogels have been prepared prepared as illustrated in scheme 2 in three steps as follow: (a) 2 g of O-CMC has been dissolved in 100 ml distilled water. The solution has been stirred at room temperature overnight. GPTMS has been added with half ratio of O-CMC to the solutions under vigorous stirring at  for 3 h to obtain (modified CMC). (b) 2 g of CMP was dissolved in 10 ml water under shaking for 2 h at room temperature. NIPAAm and BIS (2%, w/w, relative to NIPAAm) have been added to the above solution. The whole amount have been transferred to the modified CMC and stirred for 30 min. After that, the solutions have been degassed by passing nitrogen gas through it. Then, TEMED
for 3 h to obtain (modified CMC). (b) 2 g of CMP was dissolved in 10 ml water under shaking for 2 h at room temperature. NIPAAm and BIS (2%, w/w, relative to NIPAAm) have been added to the above solution. The whole amount have been transferred to the modified CMC and stirred for 30 min. After that, the solutions have been degassed by passing nitrogen gas through it. Then, TEMED  , 0.5 g prepared
, 0.5 g prepared  NPs and APS solution (2.5 %, w/v relative to monomer) have been added. Polymerization has been carried out at room temperature for 48 h. (c) The prepared nanogel was cut into small pieces and immersed in 40 ml of acidified glutaraldehyde (GA) solution for 5 h. Finally,
NPs and APS solution (2.5 %, w/v relative to monomer) have been added. Polymerization has been carried out at room temperature for 48 h. (c) The prepared nanogel was cut into small pieces and immersed in 40 ml of acidified glutaraldehyde (GA) solution for 5 h. Finally,  nanogel has been washed with water several times each one occurred overnight, then left to dry for 2 d and drying under vacuum for extra 2 d.
nanogel has been washed with water several times each one occurred overnight, then left to dry for 2 d and drying under vacuum for extra 2 d.
Scheme 2. Scheme for synthesis of  nanogel.
nanogel.
Download figure:
Standard image High-resolution image2.2.5. Incorporation of  nanogel to cotton.
nanogel to cotton.
The produced  nanogel has been homogenised under sonication for 10 min with 50 ml water. Then the produced emulsion has been applied to cotton fabric using pad-dry-cure method as follow:
nanogel has been homogenised under sonication for 10 min with 50 ml water. Then the produced emulsion has been applied to cotton fabric using pad-dry-cure method as follow:  cotton fabric was washed and dipped in the emulsion for 10 min and then padded (with 100% pick-up). Then dried at
cotton fabric was washed and dipped in the emulsion for 10 min and then padded (with 100% pick-up). Then dried at  and cured at
and cured at  . After that, all samples were washed for 45 min using non-ionic detergent (2 g l−1) at
. After that, all samples were washed for 45 min using non-ionic detergent (2 g l−1) at  , and rinsed twice in tap water.
, and rinsed twice in tap water.
2.3. Characterisation
2.3.1. Scanning electron microscopy.
The freeze-dried nanogel was fractured carefully and its morphology was observed by use of an environmental scanning electron microscope (ESEM) type Quanta 200.
2.3.2. Infrared spectrometry.
The freeze-dried nanogel was analysed using a Fourier Transform Infrared spectrophotometer (FT-IR) (VERTEX 70, Bruker, Austria) in the ranges of  .
.
2.3.3. Shape and particle size determination.
The shape and particle size were determined using transition electron microscope (TEM) and dynamic light scattering (DLS). Sample's measurements were prepared by placing a drop of colloid on carbon-coated copper grid and evaporating water at room temperature. The size distribution of the prepared nanoparticles in aqueous solution was measured with laser particle size analyzer (Nano-Sizer SZ90, Malvern instruments Ltd., UK) at  .
.
2.3.4. Calculation of degree of substitution (DS).
The degree of substitution (DS) of carboxymethylated chitosan (CMC) was determined according to BeMiller et al [31] by dissolving 0.3 g CMCs in 30 ml  (1 M) and titrating with 0.1 M aqueous
(1 M) and titrating with 0.1 M aqueous  . The DS value was calculated as follows:
. The DS value was calculated as follows:

where  and
and  are the volume and molarity of
are the volume and molarity of  , respectively,
, respectively,  was the mass of carboxymethyl chitosan (g). 161 and 58 are the molecular weights of the glucosamine and the carboxymethyl group, respectively.
was the mass of carboxymethyl chitosan (g). 161 and 58 are the molecular weights of the glucosamine and the carboxymethyl group, respectively.
3. Results and discussion
3.1. Characterization of O-carboxymethyl chitosan (O-CMC)
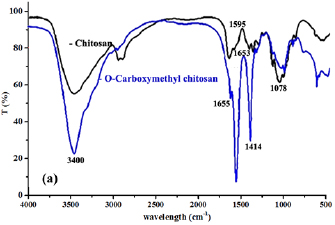
FT-IR analyses have been running to provide the structure of O-CMC. FT-IR spectra of chitosan and O- are shown in figure 1(a). The spectra of chitosan show the peaks at 1653 and  for
for  bending and at 1078 and
bending and at 1078 and  for
for  stretching [32]. It is noticed that, a shoulder peak at
stretching [32]. It is noticed that, a shoulder peak at  for both chitosan and O-CMC is due to
for both chitosan and O-CMC is due to  [33]. In addition, a stretching vibration for
[33]. In addition, a stretching vibration for  groups is presenting at
groups is presenting at  . A peak at
. A peak at  is corresponding to the
is corresponding to the  rocking of the ring and the band for amide I at
rocking of the ring and the band for amide I at  . In the spectra of O-CMC, a new peaks at
. In the spectra of O-CMC, a new peaks at  was appeared characteristic to carboxylate group asymmetrical and symmetrical stretch
was appeared characteristic to carboxylate group asymmetrical and symmetrical stretch  [34].
[34].
Figure 1. FT-IR spectra for chitosan and its carboxymethyl derivative O-CMC.
Download figure:
Standard image High-resolution image3.2. Characterization of carboxymethyl pullulan (CMP)
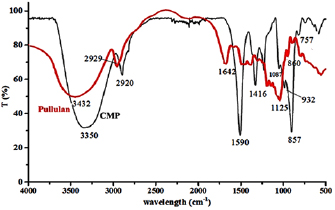
FT-IR analyses have been running to provide the structure of CMP. Figure 2 shows the FT-IR spectra of pullulan and CMP, and explain that, the characteristic absorption bands for pullulan are at 3432, 2929, 1654, 1125, 1032 1642 and  , which they relative to
, which they relative to  ,
,  ,
,  ,
,  and
and  stretching,
stretching,  bending and D-glucosidic bands respectively [29]. Furthermore, the characteristic absorption bands of CMP appears at
bending and D-glucosidic bands respectively [29]. Furthermore, the characteristic absorption bands of CMP appears at  stretching for
stretching for  of anhydroglucose units [17],
of anhydroglucose units [17],  for
for  groups,
groups,  [17] for asymmetric carboxylate group (
[17] for asymmetric carboxylate group ( ) and at
) and at  (symmetric carboxylate group (
(symmetric carboxylate group ( ) [17]. At
) [17]. At  , a characteristic stretching peak attributed to ether bond in pullulan have been observed [29]. In addition, there are three peaks for D-glucosidic which they appear at 857, 757 and
, a characteristic stretching peak attributed to ether bond in pullulan have been observed [29]. In addition, there are three peaks for D-glucosidic which they appear at 857, 757 and  [35].
[35].
Figure 2. FT-IR Spectra for pullulan and CMP.
Download figure:
Standard image High-resolution image3.3. Characterization of ZnO NPs
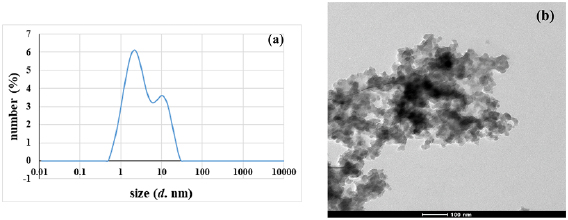
The particle size of ZnO NPs has been illustrated in figure 3. Mean particle size diameter and PDI index were all measured in solutions using dynamic light scattering (DLS). The size of the colloidal ZnO NPs and their granule metric distribution are disclosed in figure 3. As is evident the size of the particles is around 9 nm. Particle size analysis displays the presence of ZnO NPs with low  , which mean a good- quality of the colloidal suspensions [36]. Figure 3 shows TEM image which indicate that, the structure of
, which mean a good- quality of the colloidal suspensions [36]. Figure 3 shows TEM image which indicate that, the structure of  NPs are in spherical or slightly irregular shape.
NPs are in spherical or slightly irregular shape.
Figure 3. Particle size (a) and TEM micrographs (b) of  NPs.
NPs.
Download figure:
Standard image High-resolution image3.4. Characterization of  nanogel
nanogel
There are a several possible interactions between CMP, CMC, poly-NiPAAm and GPTMS when using dispersion copolymerization. Theses interactions could be covalent bonding, electrostatic and/or hydrogen interactions.
Hydrogen interactions and/or electrostatic were presence between hydroxyl groups of CMP and amine groups of poly-NiPAAm. In addition, covalent bonding is presence between hydroxyl groups in C6 of CMP and poly-NiPAAm in polymerisation process [37].
3.4.1. FT-IR, TEM and particle size analyser.
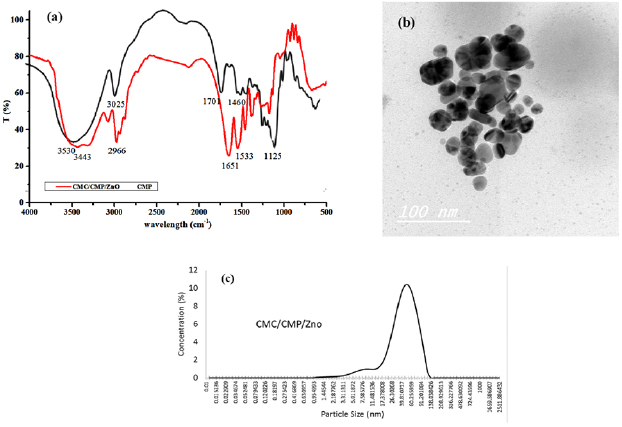
FT-IR spectra of CMP and  nanogel have been illustrated in figure 4. Sharp peaks from hydroxyl and amine groups of both CMP and CMC have been detected at
nanogel have been illustrated in figure 4. Sharp peaks from hydroxyl and amine groups of both CMP and CMC have been detected at  [17, 29]. The absorption peaks at 1460 and
[17, 29]. The absorption peaks at 1460 and  are resulted from the overlapping of the carboxylate groups from CMP and the amide I from PNIPAAm [38]. The sharp peak at
are resulted from the overlapping of the carboxylate groups from CMP and the amide I from PNIPAAm [38]. The sharp peak at  in the NIPAAm spectrum (amide II) becomes wider with lower intensity in the spectra of the nanogel which provide the presence of BIS and PNIPAAm [38]. Figure 4 shows TEM image of the
in the NIPAAm spectrum (amide II) becomes wider with lower intensity in the spectra of the nanogel which provide the presence of BIS and PNIPAAm [38]. Figure 4 shows TEM image of the  nanogel, which indicates that, the particles shape is spherical or slightly irregular. In addition, the nanogel particle size can be easily determined and provide from the TEM figure. Figure 4 shows also the particle and distribution size of
nanogel, which indicates that, the particles shape is spherical or slightly irregular. In addition, the nanogel particle size can be easily determined and provide from the TEM figure. Figure 4 shows also the particle and distribution size of  . It is clear that, the size of
. It is clear that, the size of  particles is around 50 nm. In addition there are some particles in the range of 7 nm. These differences are may be due to the different surface properties of both CMC and CMP.
particles is around 50 nm. In addition there are some particles in the range of 7 nm. These differences are may be due to the different surface properties of both CMC and CMP.
Figure 4. FT-IR (a), TEM image (b) and particle size (c) of  nanogel.
nanogel.
Download figure:
Standard image High-resolution image3.4.2. Effect of temperature and pH on  NPs.
NPs.
Particles diameter of the prepared nanogel have been investigated at pH 6.5 (figure 5), and it provides that the nanogel particles are swollen at  with 50 nm average diameter. While, at
with 50 nm average diameter. While, at  , the nanogel having 7 nm in particles size. Furthermore, the temperature was increased gradually from 27 to
, the nanogel having 7 nm in particles size. Furthermore, the temperature was increased gradually from 27 to  lead to reduce in the particle size. Above
lead to reduce in the particle size. Above  , size of nanoparticles does not vary significantly with increase in temperature. A sharp collapse at
, size of nanoparticles does not vary significantly with increase in temperature. A sharp collapse at  was observed which is corresponding normally to poly-NiPAAm homo-polymer. Observation of broad transition temperature in range of
was observed which is corresponding normally to poly-NiPAAm homo-polymer. Observation of broad transition temperature in range of  is due to presence of CMP. Ionization of CMP groups at pH 6.5 caused increasing of the hydrophilicity and imparts charge repulsion between particles.
is due to presence of CMP. Ionization of CMP groups at pH 6.5 caused increasing of the hydrophilicity and imparts charge repulsion between particles.
Figure 5. Temperature (a) and pH (b) effect on the of  nanogel particles size.
nanogel particles size.
Download figure:
Standard image High-resolution imageDynamic light scattering analyser was used to investigate the effect of pH on changing in the particle size of the prepared nanogel at  (figure 5).
(figure 5).  NPs are in swollen state at high pH (pH 10) with size of 118 nm. In addition, decreasing the pH value led to decreasing the particle size of the prepared nanogel. Swelling/de-swelling effect may be attributed to presence of CMP and/or CMC. Therefore, at high pH, nanogel particles are similar to cellulosic material (swelling behaviour). At low pH, CMP and/or CMC become soluble. Therefore, one can conclude that
NPs are in swollen state at high pH (pH 10) with size of 118 nm. In addition, decreasing the pH value led to decreasing the particle size of the prepared nanogel. Swelling/de-swelling effect may be attributed to presence of CMP and/or CMC. Therefore, at high pH, nanogel particles are similar to cellulosic material (swelling behaviour). At low pH, CMP and/or CMC become soluble. Therefore, one can conclude that  nanogel swell (basic medium) and deswell (acid medium).
nanogel swell (basic medium) and deswell (acid medium).
3.5. Incorporation of  nanogel to cotton
nanogel to cotton
Cotton fabrics have been treated with the prepared composite gel of  mixture using pad-dry-cure technique; at first—
mixture using pad-dry-cure technique; at first— nanogel were deposited onto the surface, then, at
nanogel were deposited onto the surface, then, at  forms ester, between carboxylic groups on CMP and hydroxyl groups on cellulose [39]. Two types of carboxyl groups are available for reaction from both CMC and CMP from
forms ester, between carboxylic groups on CMP and hydroxyl groups on cellulose [39]. Two types of carboxyl groups are available for reaction from both CMC and CMP from  nanogel. The crosslinking was occurs via esterification with these carboxylic groups and hydroxyl group of cotton fabric.
nanogel. The crosslinking was occurs via esterification with these carboxylic groups and hydroxyl group of cotton fabric.
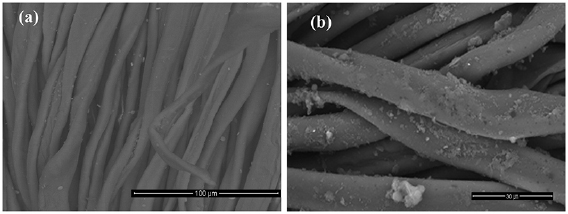
Surface morphology of both untreated and treated cotton fabrics have been illustrated in figure 6. SEM micrograph show that, Incorporation of nanogel onto surface of cotton fibre have been observed and the nanoparticles seem embedded onto the fibre surface. In addition, a thin layer of the nanogel was covers the surface of cotton fibre which have some irregular moieties observed as nanoparticles clusters.
Figure 6. SEM image of untreated (a) and treated fabric (b) with produced nanogel.
Download figure:
Standard image High-resolution image3.6. Evaluation of the antibacterial activity of functionalized gel
Two bacteria, namely Escherichia coli as Gram-negative bacteria and Staphylococcus aureus as Gram-positive bacteria have been used to investigate the antibacterial activity of the prepared composite gel against bacterial growth. As expected, from table 1 untreated fabric did not show any reduction in bacterial growth for both bacteria. In addition, treated fabric with CMP only shows poorly activity of fabric against both bacteria. That is because CMP didn't have functional group which can attack the bacteria cell wall. Presences of amino group in CMC give the fabric antibacterial activity against both bacteria under investigation.
Table 1. Antibacterial activities, mechanical and physical properties of cotton fabrics.
| Sample | Antibacterial activities | Mechanical and physical properties | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Zone of inhibition diameter in mm | Tensile strength (kg) | Elongation at a break (%) | Roughness  |
CRA | |||||||
| E. coli | S. aureus | Warp | Weft | ||||||||
| 24 h | 48 h | 72 h | 24 h | 48 h | 72 h | ||||||
| Untreated | 0 | 0 | 0 | 0 | 0 | 0 | 41 | 15 | 11.65 | 50 | 45 |
| Treated with modified CMP | 1.1 | 1.1 | 1.1 | 1 | 1 | 1 | 37.5 | 13.8 | 12.36 | 85 | 82 |
| Treated with modified CMC | 6.1 | 6.2 | 6.3 | 5.5 | 5.7 | 5.8 | 39.1 | 14.1 | 17.43 | 77 | 70 |
| Treated with modified nanogel | 14.2 | 14.8 | 14.9 | 12.1 | 12.5 | 12.6 | 39.3 | 14.5 | 14.12 | 81 | 73 |
On the other hand, the treated fabric with produced gel showed highly reactivity against both bacteria comparing with untreated and treated fabric with CMP or CMC only. As expected, presence of amine group and  NPs in the nanogel structure increasing the inhibition zone, demonstrating the antimicrobial activity of the tested treated fabric with nanogel.
NPs in the nanogel structure increasing the inhibition zone, demonstrating the antimicrobial activity of the tested treated fabric with nanogel.
The positive charge of the amine groups results in a nanogel structure and presence of  NPs were cooperate with the transcendently anionic lipopolysaccharides of the Gram-negative cell surface advancing hindering the vehicle of supplements into the cells or the spillage of intracellular segments [40, 41].
NPs were cooperate with the transcendently anionic lipopolysaccharides of the Gram-negative cell surface advancing hindering the vehicle of supplements into the cells or the spillage of intracellular segments [40, 41].
3.7. Mechanical and physical characterization
Mechanical and physical properties of untreated and treated cotton fabrics have been investigated and the parameters have been illustrated in table 1. The results provide that, the finishing treatment show a significant decreasing in both tensile strength and elongation at a break of the treated fabrics comparing to untreated one. In addition, roughness properties show a slight increase in its values, which due to the deposition of nanogel and  NPs onto the surface of cotton fabrics. The produced film onto the cotton surface led to improve the crease recover angel (CRA) of all treated fabrics, and treated fabrics with nanogel shows highly value comparing with the other treated fabric with CMC or CMP only.
NPs onto the surface of cotton fabrics. The produced film onto the cotton surface led to improve the crease recover angel (CRA) of all treated fabrics, and treated fabrics with nanogel shows highly value comparing with the other treated fabric with CMC or CMP only.
3.8. Effect of temperature and pH on the cotton fabrics
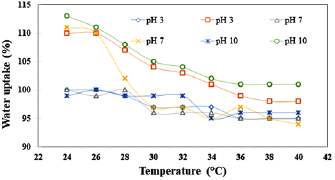
Effect of both temperature and pH on the treated and untreated cotton fabrics have been investigated by measuring water uptake at varies temperatures range from 24 to  and different pH (3, 7 and 10) (figure 7). Water uptake for untreated fabrics was almost constant (95% to 100%) for all measured pH at ranged temperature.
and different pH (3, 7 and 10) (figure 7). Water uptake for untreated fabrics was almost constant (95% to 100%) for all measured pH at ranged temperature.
Figure 7. Effect of temperature and pH on the water uptake for cotton fabrics.
Download figure:
Standard image High-resolution imageThe water uptake at pH 7 was constant while increasing temperature from 24 to  led to decreasing of water uptake from 111 to 97% whenever increasing temperature from 26 till
led to decreasing of water uptake from 111 to 97% whenever increasing temperature from 26 till  . In addition, water uptake remained constant with further temperature increase. In acidic medium (pH 3), a significant weaker response compared to values at pH 7, but it shows same behaviour. On the other hand, at alkaline medium (pH 10), water uptake was in higher values than those values at pH 3 or 7. Furthermore, when the nanogel was in collapsed state, water uptake was always lower comparing to untreated fabric. This phenomenon confirmed that, in collapsed state, the nanogel surface becomes hydrophobic and increased hydrophobicity of treated fabric. Raising the temperature cause that, PNIPAAm undergoes collapse transitions [16, 42].
. In addition, water uptake remained constant with further temperature increase. In acidic medium (pH 3), a significant weaker response compared to values at pH 7, but it shows same behaviour. On the other hand, at alkaline medium (pH 10), water uptake was in higher values than those values at pH 3 or 7. Furthermore, when the nanogel was in collapsed state, water uptake was always lower comparing to untreated fabric. This phenomenon confirmed that, in collapsed state, the nanogel surface becomes hydrophobic and increased hydrophobicity of treated fabric. Raising the temperature cause that, PNIPAAm undergoes collapse transitions [16, 42].
At the point when the chain is warmed up, all the hydrogen bonds were broken, subsequent the chain in the collapse sharp. The captured water molecules through hydrogen bonds in nanogel are released. Moreover, in light of its hydrophilic nature influenced by pH, incorporation of both CMP and CMC expected that, bearing many hydroxyl groups (hydrophilic nature) into the PNIPAAm network.
At lower pH and temperature ( ), the particles of nanogels were in marginally collapsed state. Thus, the primary water uptake was investigated at
), the particles of nanogels were in marginally collapsed state. Thus, the primary water uptake was investigated at  for pH 3 is lower than its value at pH 7. Notwithstanding, above
for pH 3 is lower than its value at pH 7. Notwithstanding, above  for all measured pH, the water uptake was almost same. Nevertheless, this procedure is being backed off because of CMP, CMC and PNIPAAm chains present in the nanogel, which keeps water molecules inside the nanogel network.
for all measured pH, the water uptake was almost same. Nevertheless, this procedure is being backed off because of CMP, CMC and PNIPAAm chains present in the nanogel, which keeps water molecules inside the nanogel network.
Furthermore, from another point of view, in the figure 5(a), which indicates the effect of the temperature on the size of  nanogel particles, it is obvious that the nanogel start decomposing (collapse) at
nanogel particles, it is obvious that the nanogel start decomposing (collapse) at  and completely decomposing at
and completely decomposing at  with particle size around 9 nm (particle size of 9 nm is the size of only
with particle size around 9 nm (particle size of 9 nm is the size of only  ). So, when then the fabric coated with the nanogel and applying high temperature for drying and curing processes until
). So, when then the fabric coated with the nanogel and applying high temperature for drying and curing processes until  the nanogel around
the nanogel around  NPs is completely decomposed and make a thin film around the fibre surface. This coated film covered the whole surface and have the nanoparticle embedded through its network. In addition, presence of the crosslinker during these processes allows the nanogel to be chemically bonded with cellulose chains and the nanoparticle was rented in the gaps between cellulose chains and nanogel network.
NPs is completely decomposed and make a thin film around the fibre surface. This coated film covered the whole surface and have the nanoparticle embedded through its network. In addition, presence of the crosslinker during these processes allows the nanogel to be chemically bonded with cellulose chains and the nanoparticle was rented in the gaps between cellulose chains and nanogel network.
4. Conclusion
In this study hybrid nanogel containing CMP, O-CMC, PNIPAAm and silane have been synthesized. CMP, O-CMC,  NPs have been prepared and characterised using FT-IR, NMR, SEM, and particle size. Characterisation provides the chemical modification and the particle size of
NPs have been prepared and characterised using FT-IR, NMR, SEM, and particle size. Characterisation provides the chemical modification and the particle size of  NPs. Further investigation was done for the prepared
NPs. Further investigation was done for the prepared  nanogel through FT-IR, TEM and particle size analyser. Effect of temperature and pH on
nanogel through FT-IR, TEM and particle size analyser. Effect of temperature and pH on  nanoparticles was also measured and provides that, the nanogel particles are swollen at
nanoparticles was also measured and provides that, the nanogel particles are swollen at  with 50 nm average diameter, with final conclusion, the
with 50 nm average diameter, with final conclusion, the  nanogel swell (basic medium) and deswell (acid medium). In addition, there was an important characteristic for the prepared nanogel, as to down of the human body temperature, the nanogel capacity to swell and deswell is improved.
nanogel swell (basic medium) and deswell (acid medium). In addition, there was an important characteristic for the prepared nanogel, as to down of the human body temperature, the nanogel capacity to swell and deswell is improved.
Furthermore, incorporation of  nanogel to cotton fabric has been investigated. The
nanogel to cotton fabric has been investigated. The  NPs seem to be embedded onto the fibre surface. In addition, a thin layer of the nanogel was covers the surface of cotton fibre with presence of some irregular clusters of nanoparticles. The prepared gel showed antimicrobial activity toward S. aureus and E. coli attributed to the presence of amino groups and silicon compound in the polymeric gel network. Mechanical and physical properties of cotton fabrics were also investigated. Effect of temperature and pH on the cotton fabrics were investigated by measuring water uptake at different pH and temperatures.
NPs seem to be embedded onto the fibre surface. In addition, a thin layer of the nanogel was covers the surface of cotton fibre with presence of some irregular clusters of nanoparticles. The prepared gel showed antimicrobial activity toward S. aureus and E. coli attributed to the presence of amino groups and silicon compound in the polymeric gel network. Mechanical and physical properties of cotton fabrics were also investigated. Effect of temperature and pH on the cotton fabrics were investigated by measuring water uptake at different pH and temperatures.
Acknowledgments
This project was supported financially by the Science and Technology Development Fund (STDF), Egypt, Grant No. 5473). Furthermore, the authors also gratefully grateful acknowledge to National Research Centre (NRC) for facilities provided.