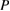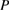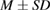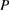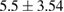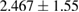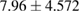Abstract
The objective of this work is to prepare and verify the antimicrobial properties of tissue conditioner containing silver doped bioactive glass nanoparticles (Ag-BG NPs) in vitro against three oral pathogens. A novel bioactive glass (BG) nanoparticles (NPs) with different Ag weight percentages of 0.6, 1.2, 3 and 4 wt% was prepared via a modified sol-gel method. Characterization of Ag-BG NPs was carried out using transmission electron microscopy (TEM), Fourier transformer infrared spectrophotometer (FTIR). Antimicrobial activities of the NPs were estimated. Also,  ions release was measured using an inductively coupled plasma optical emission spectroscopy (ICP/OES). The 4% Ag-BG NPs was incorporated into the tissue conditioner (TC) for the preparation of the composite specimens (TC/Ag BG 4%). The dispersion of the NPs was evaluated using SEM/EDX.
ions release was measured using an inductively coupled plasma optical emission spectroscopy (ICP/OES). The 4% Ag-BG NPs was incorporated into the tissue conditioner (TC) for the preparation of the composite specimens (TC/Ag BG 4%). The dispersion of the NPs was evaluated using SEM/EDX.  ion release and antimicrobial activities were evaluated. Finally, cell cytotoxicity was assessed using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay on normal human fibroblast (BJ1). Data analysis was performed using one-way ANOVA, and Tukey's post hoc test. The Ag-BG NPs (4%) exhibited the highest statistical significant mean inhibition value against selected pathogens. A novel Ag-BG NPs with antimicrobial properties with gradual release of
ion release and antimicrobial activities were evaluated. Finally, cell cytotoxicity was assessed using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay on normal human fibroblast (BJ1). Data analysis was performed using one-way ANOVA, and Tukey's post hoc test. The Ag-BG NPs (4%) exhibited the highest statistical significant mean inhibition value against selected pathogens. A novel Ag-BG NPs with antimicrobial properties with gradual release of  were developed. However, the antimicrobial activity was lost when it was incorporated into the tissue conditioner.
were developed. However, the antimicrobial activity was lost when it was incorporated into the tissue conditioner.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 3.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
The use of soft liners is an indispensable aid in the treatment of patient wearing removable prostheses. Being resilient, soft liners compensate the reduced thickness of oral mucosal and equilibrate the masticatory stresses; thus, minimizing the incidence of pressure points, traumatic ulcerations and pain during mastication [1, 2]. Furthermore, their application is recommended in cases requiring maxillofacial prosthesis; As they alleviate patient's pain and discomfort without causing trauma to the soft tissues which is sensitive and easily irritated [3].
Two types of soft liners are available: permanent liners and temporary liners which are known as tissue conditioner. Currently, most of the available permanent liners made of silicon and methyl/ethyl methacrylate cross-linked amorphous polymers [4]. On the other hand, tissue conditioners consist of un-crosslinked amorphous polymers. They are supplied in the form of powder and liquid. The main constituent of the powder is poly (ethyl methacrylate) or a related copolymer. The liquid is a mixture of a plasticizer as butyl phthalyl, butyl glycolate, dibutyl phthalate, dibutyl sebacate, and ethyl alcohol. Other material based on poly (butyl methacrylate) or a related copolymer does not contain ethyl alcohol in the liquids [5].
One of the major problems associated with the use of soft liners is inevitable biofilm formation and subsequent microbial colonization [6]. Microorganisms as Candida albicans (C. albicans), Streptococci mutans (S. mutans) and Staphylococcus aureus (S. aureus) have been isolated from patients wearing removal prostheses [7]. Such pathogens are capable of initiating infectious diseases as denture stomatitis, pharyngeal and respiratory infections particularly in elderly and medically compromised individuals [8–11]. Although, different mechanical and chemical cleansing methods are available, none of them were efficient to prevent biofilm formation or to eradicate microorganisms from lining materials. Besides, these methods can cause substantial damage to liners affecting their durability [12]. Therefore, the maintenance of integrity of soft liners and the prevention of the microbial accumulation are of great importance, the search for liner with antimicrobial characteristics is an important demand for the maintenance of removable prostheses [13].
Silver is well-known for its antimicrobial characteristic against a broad spectrum of bacteria and fungi [14]. With the development of nanomaterials, silver nanoparticles (AgNPs) demonstrated potent antimicrobial characteristics for treating infectious diseases including drug-resistant ones [15, 16]. One of the critical aspects of silver NPs in biomedical practice is its precipitation that could result in toxic effects. Therefore, the need for silver carrier as glass nanoparticles is desirable prerequisite. Silver doped glasses (Ag-BG) are reported to be very effective bactericidal agents compared to those containing silver in other oxidation states (+2 or +3) [17, 18]. The present study aimed to investigate the biocidal activity of tissue conditioner containing silver doped bioactive glass nanoparticles against three pathogens: Candida albicans, Streptococcus mutans and Staphylococcus aureus.
2. Materials and methods
2.1. Preparation and characterization of Ag-doped bioactive glass nanoparticles
Novel Ag-doped bioactive glass nanoparticles (Ag-BG NPs) with four weight percentages of Ag ions were prepared via a modified sol-gel method [19]. Silver oxide (Ag2O) was added to glass composition at the expense of the calcium oxide (CaO) as expressed by the general formula:  , all in wt% and
, all in wt% and  and 4%. The prepared glass compositions were represented in table 1. The particle size was studied using transmission electron microscopy (TEM, JEM-HR-2100, Japan). The infrared spectra were carried out using a Fourier transformer infrared (FTIR) spectrophotometer (model FT/IR-6100 type A, USA). Nitrogen adsorption-desorption isotherms were determined by means of a high-speed gas sorption analyzer (NOVA 2000 series, Chromatic, UK). The Brunauer–Emmett–Teller (BET) method was employed to determine the specific surface areas. In addition, pores volume, average pore diameters and pore-size distribution were also estimated.
and 4%. The prepared glass compositions were represented in table 1. The particle size was studied using transmission electron microscopy (TEM, JEM-HR-2100, Japan). The infrared spectra were carried out using a Fourier transformer infrared (FTIR) spectrophotometer (model FT/IR-6100 type A, USA). Nitrogen adsorption-desorption isotherms were determined by means of a high-speed gas sorption analyzer (NOVA 2000 series, Chromatic, UK). The Brunauer–Emmett–Teller (BET) method was employed to determine the specific surface areas. In addition, pores volume, average pore diameters and pore-size distribution were also estimated.
Table 1. Different compositions of bioactive glass in weight%.
| Sample | Bioactive glass compositions (wt%) | ||
|---|---|---|---|
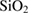 |
CaO | 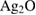 |
|
| Ag-BG 0.6 | 80 | 19.4 | 0.6 |
| Ag-BG 1.2 | 80 | 18.8 | 1.2 |
| Ag-BG 3 | 80 | 17 | 3 |
| Ag-BG 4 | 80 | 16 | 4 |
2.2. Determination of antimicrobial activities of Ag-BG NPs
The antimicrobial characteristics of Ag-BG NPs were assessed using well diffusion method.
2.2.1. Microorganisms.
The microorganisms tested were: C. albicans (ATCC10231), S. aureus (ATCC6538) and S. mutans. The latter were isolated and identified by the Microbiology Lab, Department of Microbiology and Dairy Science, National Research Centre, where S. mutans was obtained by swabbing all surfaces of the teeth of high caries index patient (National Research Centre ethical committee approval registration no: 13-028), using sterile cotton tipped swab (Q-tips, Dermacea, Sherwood Medical, St Louis, USA). The swab was placed in a 5 ml sterile container containing 2 ml of phosphate-buffered saline (PBS, pH 7.4). Then swab in PBS was placed in a vortex (Thermolyne Maxi Mix II, Iowa, and USA) for five minutes to dislodge bacteria followed by Serial dilutions to form dilution at  ,
,  and
and  using an automatic micropipette. The selective media mitis salivarius-bacitracin agar (MSB, Fluka and catalogue No. 01337, Sigma-Aldrich, USA) was used to isolate and grow S. mutans.
using an automatic micropipette. The selective media mitis salivarius-bacitracin agar (MSB, Fluka and catalogue No. 01337, Sigma-Aldrich, USA) was used to isolate and grow S. mutans.
Each of S. aureus and S. mutans were grown in test tubes containing 10 ml of treptone soya broth (TSB, Difco, Detroit, MI USA). Then, they were incubated at  for 24 h to grow. Each culture was diluted into fresh media until a concentration of
for 24 h to grow. Each culture was diluted into fresh media until a concentration of  was reached and used as a working microbial solution [20].
was reached and used as a working microbial solution [20].
For the C. albicans, the fungal strain was kept on sabouraud broth medium (1% peptone, 4% glucose and adjusted to pH 5.8) to grow at temperature range of  for 48 h. Then, culture was diluted into a fresh media until a concentration
for 48 h. Then, culture was diluted into a fresh media until a concentration  was reached and used as a working microbial solution.
was reached and used as a working microbial solution.
2.2.2. Microbiological assay.
Aqueous suspensions of the four glass samples (Ag-BG 0.6, Ag-BG 1.2, Ag-BG 3 and Ag-BG 4) were prepared ( ) [21]. For S. mutans, S. aureus, and C. albicans,
) [21]. For S. mutans, S. aureus, and C. albicans,  of each working microbial solutions was spread evenly over a nutrient agar plate. Then, six wells were cut using sterile 5 mm cork-borer. Four of these wells were filled with
of each working microbial solutions was spread evenly over a nutrient agar plate. Then, six wells were cut using sterile 5 mm cork-borer. Four of these wells were filled with  of the previously prepared Ag-BG suspensions (
of the previously prepared Ag-BG suspensions ( in water). The fifth well was filled with
in water). The fifth well was filled with  saline water as a negative control and the sixth one was filled with
saline water as a negative control and the sixth one was filled with  of mycostatin (Nystatin
of mycostatin (Nystatin  suspension, Glaxo Smith Kline, Giza, Egypt) as a positive control against C. albicans, while 0.2% chlorhexidine digluconate (CHX, Sigma Aldrich, USA) was used as a positive control against S. mutans and S. aureus [22].
suspension, Glaxo Smith Kline, Giza, Egypt) as a positive control against C. albicans, while 0.2% chlorhexidine digluconate (CHX, Sigma Aldrich, USA) was used as a positive control against S. mutans and S. aureus [22].
Following incubation period specified for tested strains, the plates were examined visually for the inhibitions zones. Each assay was repeated three times independently (triplicate). Ag-BG NPs composition that showed the highest inhibition zone values was selected and used for the preparation of the Ag-BG tissue conditioner composite specimens.
2.3.  ions release from Ag-BG 4 NPs powder
ions release from Ag-BG 4 NPs powder
 ions released from Ag-BG 4 sample was evaluated using an inductively coupled plasma optical emission spectroscopy (ICP/OES, Model Agilent 5100VDV, Germany) with a detection limit of
ions released from Ag-BG 4 sample was evaluated using an inductively coupled plasma optical emission spectroscopy (ICP/OES, Model Agilent 5100VDV, Germany) with a detection limit of  .
.
50 mg of Ag-BG 4 NPS was immersed in 100 ml of deionized water in polypropylene tubes at  under agitation for different periods: 2 h, 4 h, 24 h, 48 h, 4 days, 5 d, 6 d, 7 d,8 d, 9 d and 10 d.
under agitation for different periods: 2 h, 4 h, 24 h, 48 h, 4 days, 5 d, 6 d, 7 d,8 d, 9 d and 10 d.
2.4. Preparation of tissue conditioner (TC) composite containing the Ag-BG 4 (TC/Ag BG 4%)
Tissue conditioner (Co soft, GC, USA) was supplied in the form of powder and liquid. For the preparation of the composite discs, different weight ratios (3%, 4%, 6%, 12.5%, 25% and 35%) of Ag-BG 4 NPs were used. Ag-BG 4 NPs particles was added to the monomer liquid in order to reduce the agglomeration of the NPs and to ensure its appropriate dispersion [23]. Disc specimens ( ) were prepared by adding the powder to liquid (6 gm:5 ml) then manually mixed following the manufacturers' instruction. Mixture was packed into specifically designated metal molds, sandwiched between two glass slabs and allowed to set. Ag-BG 4 NPs 35% weight ratio was the proper to be added without affecting consistency of the tissue conditioner.
) were prepared by adding the powder to liquid (6 gm:5 ml) then manually mixed following the manufacturers' instruction. Mixture was packed into specifically designated metal molds, sandwiched between two glass slabs and allowed to set. Ag-BG 4 NPs 35% weight ratio was the proper to be added without affecting consistency of the tissue conditioner.
A total of 19 composite disc specimens were prepared. All specimens were sterilized using ultraviolet light to prevent the bacterial contamination [24]. Similarly, six tissue conditioner disc specimens were prepared and used as control. The specimens used for  was kept immersed in deionized water.
was kept immersed in deionized water.
The microstructure of the cross-section of composite containing Ag-BG 4 was examined by means of SEM/EDX (SEM Model Quanta FEG 250, Holland) to confirm the presence of the Ag-doped glass nanoparticles within its matrix.
2.5. Evaluation of antimicrobial activity of composite
The antimicrobial activity of the composite and its elutes (leach out) were evaluated against same strains using agar disk diffusion [25] and well diffusion methods respectively. Specimen elutes materials were obtained by keeping the specimens immersed in 100 ml of double deionized water for two days, two weeks and four weeks at  in a shaking incubator (Innova 43, New Brunswick, USA).
in a shaking incubator (Innova 43, New Brunswick, USA).
Using the agar disk diffusion method,  of each previously prepared working microbial solution were spread evenly over a nutrient. A 5 mm filter paper discs (Whatman, USA) containing 0.2% chlorhexidine and mycostatin (positive control) were prepared. The filter paper discs and composite specimens were placed onto the agar surface. For elutes, well diffusion method was used. Four wells were cut. Three wells were filled with
of each previously prepared working microbial solution were spread evenly over a nutrient. A 5 mm filter paper discs (Whatman, USA) containing 0.2% chlorhexidine and mycostatin (positive control) were prepared. The filter paper discs and composite specimens were placed onto the agar surface. For elutes, well diffusion method was used. Four wells were cut. Three wells were filled with  of elutes material and the fourth one was either filled with
of elutes material and the fourth one was either filled with  of 0.2% chlorhexidine digluconate or the mycostatin. The agar plates were incubated and cultured following the same procedures as described before for the tested strains. The diameters of inhibition zones formed around specimens, elutes and positive control were measured in mm.
of 0.2% chlorhexidine digluconate or the mycostatin. The agar plates were incubated and cultured following the same procedures as described before for the tested strains. The diameters of inhibition zones formed around specimens, elutes and positive control were measured in mm.
2.6. Determination of  ions release from TC/BGAg4 composite
ions release from TC/BGAg4 composite
In polypropylene tubes, composites were immersed in 100 ml of deionized water at  under agitation. The released
under agitation. The released  ions were measured after two days, two weeks and four weeks using an inductively coupled plasma optical emission spectroscopy (ICP/OES, Model Agilent 5100VDV, Germany).
ions were measured after two days, two weeks and four weeks using an inductively coupled plasma optical emission spectroscopy (ICP/OES, Model Agilent 5100VDV, Germany).
2.7. Cytotoxicity
The potential cytotoxicity effect of TC/BGAg4 composite was determined using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay test. The test was conducted on human normal fibroblast cell (BJ1) in Bioassay-Cell Culture Laboratory (National Research Centre, Cairo, Egypt). All test steps were performed in a Laminar flow cabinet biosafety class II level (Baker, SG403INT, Sanford, ME, USA).
2.7.1. Sample preparation for cytotoxic test.
Both composite and control specimens (tissue conditioner only) were immersed in 100 ml of Dulbecco's modified eagle's medium Ham's F-12 (DMEM/F12) cell culture medium (1:1 MIX Lonza, Veries, Belgium) for 2 d and 4 weeks. Cytotoxicity of the specimen elutes were assessed, after each immersion period. Specimens were examined in three independent tests.
2.7.2. MTT assay.
BJ1 cells were suspended in DMEM/F12, 1% antibiotic-antimycotic mixture ( potassium penicillin,
potassium penicillin,  streptomycin sulfate and
streptomycin sulfate and  amphotericin B) and 1% L-glutamine at
amphotericin B) and 1% L-glutamine at  under 5%
under 5%  . Cells were batch cultured for 10 d and seeded at concentration of
. Cells were batch cultured for 10 d and seeded at concentration of  in a new growth medium into 96-well microtiter plastic plates at
in a new growth medium into 96-well microtiter plastic plates at  for 24 h under 5%
for 24 h under 5%  using water jacketed carbon dioxide incubator (Sheldon, TC2323, Cornelius, OR, USA).
using water jacketed carbon dioxide incubator (Sheldon, TC2323, Cornelius, OR, USA).
After 24 h, the media was replaced with equal volumes of elute materials from both composite specimens' and tissue conditioning. For negative control, cells were incubated in DMEM/F12 alone. Whereas, positive control  of a natural agent of known cytotoxic effects (100% lethality) under the same conditions [26, 27] was used.
of a natural agent of known cytotoxic effects (100% lethality) under the same conditions [26, 27] was used.
After 48 h of incubation, the medium was replaced with 40 µl MTT salt (2.5 µg ml−1) and incubated for further 4 h at  under 5%
under 5%  . To stop the reaction and dissolve the formed crystals,
. To stop the reaction and dissolve the formed crystals,  of the 10% sodium dodecyl sulphate (SDS) in deionized water was added then incubated overnight at
of the 10% sodium dodecyl sulphate (SDS) in deionized water was added then incubated overnight at  . Absorbance was measured using a microplate multi-well reader (Bio-Rad Laboratories Inc., model 3350, Hercules, California, USA) at 595 nm and a reference wavelength of 620 nm. A statistical significance was tested between specimens and negative control using independent
. Absorbance was measured using a microplate multi-well reader (Bio-Rad Laboratories Inc., model 3350, Hercules, California, USA) at 595 nm and a reference wavelength of 620 nm. A statistical significance was tested between specimens and negative control using independent  by SPSS 11 program. The percentage of change in viability was calculated according to the following formula:
by SPSS 11 program. The percentage of change in viability was calculated according to the following formula:

2.8. Statistical analysis
Data were tabulated as mean (M) and standard deviations (SD). For microbial assays and ion release test statistical analysis were performed using IBM® SPSS® (SPSS Inc., IBM Corporation, NY, USA) statistics version 20 for windows. One-way analysis of variance (ANOVA) was followed by Tukey's post hoc test for multiple comparisons and  value was set at
value was set at  . Regarding cytotoxic effects independent
. Regarding cytotoxic effects independent  was used.
was used.
3. Results
3.1. Characterization of Ag-doped bioactive glass nanoparticles (Ag-BG NPs)
3.1.1. Transmission electron microscopy.
TEM images of all glass compositions are presented in figure 1. Cluster of glass particle of sizes less than 100 nm was revealed. Hence the nanosized particles were proved to be formed.
Figure 1. TEM images of the Ag-doped bioactive glass nanoparticles: (a) Ag-BG 0.6, (b) Ag-BG 1.2, (c) Ag-BG 3, and (d) Ag-BG 4.
Download figure:
Standard image High-resolution image3.1.2. Fourier transformer infrared spectrophotometry.
The FTIR spectra of the Ag-BG 0.6, Ag-BG 1.2, Ag-BG 3 and Ag-BG 4 are shown in figures 2((a)–(c) and (d), respectively). The bands sited within the range of wavenumber  were associated to the Si−O−Si asymmetric stretching vibration, whereas the bands found in the range of 725–800 cm−1 were linked to the Si−O−Si symmetric stretching vibration. Furthermore, the bands detected in the range of
were associated to the Si−O−Si asymmetric stretching vibration, whereas the bands found in the range of 725–800 cm−1 were linked to the Si−O−Si symmetric stretching vibration. Furthermore, the bands detected in the range of  were correlated to the Si−O−Si bending mode [19].
were correlated to the Si−O−Si bending mode [19].
Figure 2. FTIR spectra of (a) Ag-BG 0.6, (b) Ag-BG 1.2, (c) Ag-BG 3 and (d) Ag-BG 4.
Download figure:
Standard image High-resolution image3.1.3. Textural analysis.
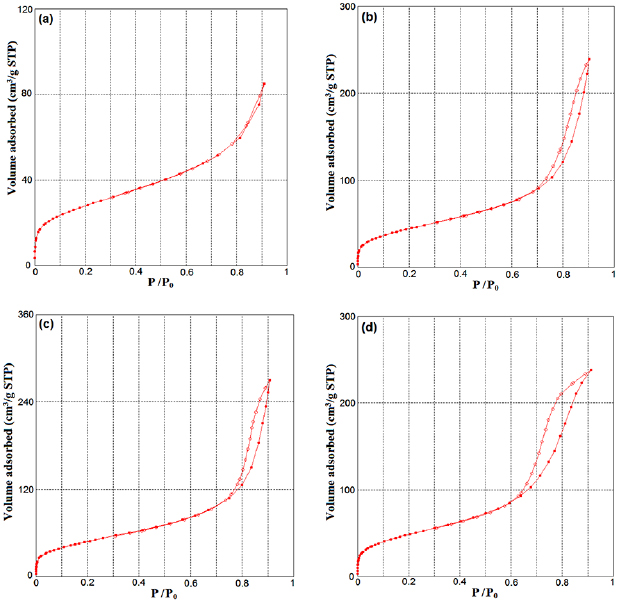
The textural properties of Ag-BG NPs determined from the nitrogen  adsorption and desorption isotherms were demonstrated in figure 3. Corresponding to the IUPAC the glass compositions were identified as type IV, which is descriptive of the mesoporous materials of 2−50 nm [28]. The hysteresis loop of this isotherm revealed different paths for desorption and adsorption branches at relatively high pressure
adsorption and desorption isotherms were demonstrated in figure 3. Corresponding to the IUPAC the glass compositions were identified as type IV, which is descriptive of the mesoporous materials of 2−50 nm [28]. The hysteresis loop of this isotherm revealed different paths for desorption and adsorption branches at relatively high pressure  .
.
Figure 3.  adsorption-desorption isotherms measured for all Ag-doped bioactive glass nanoparticles: (a) Ag-BG 0.6, (b) Ag-BG 1.2, (c) Ag-BG 3 and (d) Ag-BG 4.
adsorption-desorption isotherms measured for all Ag-doped bioactive glass nanoparticles: (a) Ag-BG 0.6, (b) Ag-BG 1.2, (c) Ag-BG 3 and (d) Ag-BG 4.
Download figure:
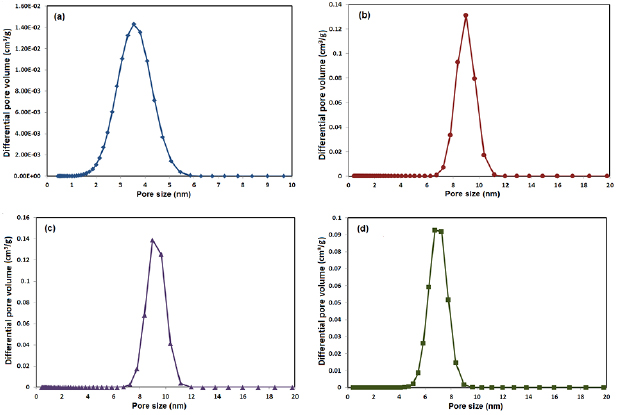
Standard image High-resolution imageThe pore size distribution curves obtained for all Ag-doped glass nanoparticles (Ag-BG 0.6, Ag-BG 1.2, Ag-BG 3 and Ag-BG 4) by employing the density functional theory (DFT) method were presented in figures 4((a)–(c) and (d) respectively).
Figure 4. Pore size distribution curves obtained for (a) Ag-BG 0.6, (b) Ag-BG 1.2, (c) Ag-BG 3 and (d) Ag-BG by employing the DFT method.
Download figure:
Standard image High-resolution imageAll samples show narrow and mono-modal pore size distribution in the mesoporous range. The specific surface area (SSA) was calculated using Brunauer–Emmett–Teller (BET) method. The textural characteristics of the Ag-doped glass nanoparticles are listed in table 2.
Table 2. Textural characteristics of Ag-doped bioactive glass nanoparticles.
| Samples | Modal pore size  |
Total pore volume  |
Specific surface area 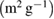 |
|---|---|---|---|
| Ag-BG 0.6 | 3.540 | 0.1016 | 98.15 |
| Ag-BG 1.2 | 8.996 | 0.3636 | 160.18 |
| Ag-BG 3 | 8.996 | 0.3978 | 170.57 |
| Ag-BG 4 | 6.752 | 0.3496 | 171.10 |
3.2. Antimicrobial activity
3.2.1. Antimicrobial activity of Ag-BG NPs.
The glass nanoparticles samples (Ag-BG 1.2, Ag-BG 3 and Ag-BG 4) showed antimicrobial activity against tested strains (tables 3–5). On other hand, the Ag-BG 0.6 sample could not induce an inhibition zone. The NPs were more efficient against S. mutans followed by C. albicans and at last the S. aureus.
Table 3. An inhibition zone exhibited by different concentrations of Ag-doped bioactive glass NPs against C. albicans growth.
| Sample | 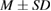 |
|---|---|
| Ag-BG 0.6 | Nil |
| Ag-BG 1.2 | 7.33a ± 2.08 |
| Ag-BG 3 | 9.33a ± 2.1 |
| Ag-BG 4 | 17.33b ± 1.53 |
| Saline | Nil |
| Mycostatin | 12.33c ± 1.5 |
Table 4. An inhibition zone exhibited by different concentrations of Ag doped bioactive glass NPs against S. mutans growth.
| Sample | 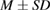 |
|---|---|
| Ag-BG 0.6 | Nil |
| Ag-BG 1.2 | 20a ± 1.63 |
| Ag-BG 3 | 24.25b ± 0.96 |
| Ag-BG 4 | 26.5c ± 1 |
| Saline | Nil |
| Chlorhexidine | 31.75d ± 1.26 |
3.2.2. Antimicrobial activity of composite specimens.
The Ag-BG NPs 4% specimens did not show any inhibitory effect against the selected strains, while the positive control (0.2% chlorhexidine digluconate) induced an inhibition zone.
The concentration Ag-BG 4 demonstrated the highest mean values against the three test stains compared to other Ag-BG NPs and it was statistically significant ( ).
).
3.3. Characterization of TC/Ag-BG 4 composite
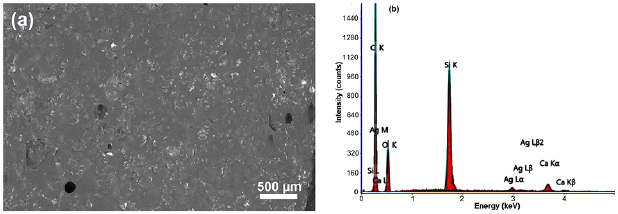
Figure 5 shows the SEM/EDX micrograph of Ag-BG 4 composite cross-section. It demonstrates that the glass NPs were distributed throughout the matrix. EDX analysis revealed silica, calcium and silver peaks which are the main components of the glass NPs.
Figure 5. (a) SEM microphotograph of cross-section of tissue TC/Ag-BG 4 composite, and (b) EDX spectrum of TC/BG 4 composite.
Download figure:
Standard image High-resolution image3.4. Silver ions release
The release of the  from the Ag-BG 4 NPs sample was measured up to the eighth day attaining the maximum amount in the fourth day (
from the Ag-BG 4 NPs sample was measured up to the eighth day attaining the maximum amount in the fourth day ( ) as shown in figure 6. Afterwards, there was a gradual decrease in the
) as shown in figure 6. Afterwards, there was a gradual decrease in the  concentration with detection limit of (
concentration with detection limit of ( ).
).
Figure 6. Ag ion release from Ag-BG 4 aqueous suspension.
Download figure:
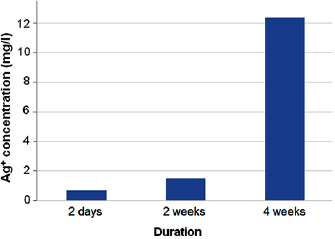
Standard image High-resolution imageIn contrast, the composite specimens showed gradual Ag ion release till the end of the fourth weeks (figure 7). Statistical analysis revealed that there was no significant difference between two days and two weeks ( ), but there was a significant difference after four weeks (
), but there was a significant difference after four weeks ( ).
).
Figure 7. Ag ion release from the TC/Ag-BG 4 composite specimens.
Download figure:
Standard image High-resolution image3.5. Cytotoxicity
Tissue conditioner (TC) showed no cytotoxic effect after two days, but there was negligible reduction in cell viability after 4 weeks (2.46%). This difference was statistically significant ( ). On the other hand, the composite (TC/Ag-BG 4) shows slight reduction in cell viability after two days (5.5%) and four weeks (7.96%) and it was statistically insignificant (
). On the other hand, the composite (TC/Ag-BG 4) shows slight reduction in cell viability after two days (5.5%) and four weeks (7.96%) and it was statistically insignificant ( ). Comparing the effect of TC and composite TC/Ag-B on the cell viability, there was statistical significant difference after two days (
). Comparing the effect of TC and composite TC/Ag-B on the cell viability, there was statistical significant difference after two days ( ) and insignificant difference after four weeks (
) and insignificant difference after four weeks ( ). From this result (table 6) it is apparent that Ag-BG 4 is nontoxic (biocompatible) as their cell viability 92%.
). From this result (table 6) it is apparent that Ag-BG 4 is nontoxic (biocompatible) as their cell viability 92%.
4. Discussion
Ag NPs is one of the most extensively investigated nano-material for its bactericidal and fungicidal characteristics as reported in several previous and recent studies [29–32].
Several studies reported incorporation of Ag NPs in polymeric dental materials such as denture base materials, soft liners and orthodontic adhesives [33–36]. Although, it adds antimicrobial benefit, the optimal quantity of Ag NPs is crucial, to avoid the cytotoxic risks owing to the precipitation of the Ag NPs [37]. Bioactive glasses nanoparticles containing  could be a better alternative to Ag NPs alone as
could be a better alternative to Ag NPs alone as  ions form a fundamental part of the glassy matrix.
ions form a fundamental part of the glassy matrix.
In the present study the antimicrobial activity of the prepared Ag-BG NPs powder was tested against three oral pathogens: C. albicans, S. mutans and S. aureus. The formation of inhibition zones confirmed the antimicrobial activity of bioactive glass containing  and the increased antimicrobial activity was concurrent with the concentration. Such finding denotes the release of
and the increased antimicrobial activity was concurrent with the concentration. Such finding denotes the release of  from the glass particle surface [38], its dissemination into the agar media and its inhibitory effects upon tested pathogens growth. Similar antimicrobial findings exhibited by the glass NPs containing ZnO was reported in the literature [21]. The release of Ag from the bioactive glass of 4% concentration was further confirmed using the ion release test. Although the maximum Ag ions release value was detected in the fourth day, there was a gradual release till the eighth day. Consequently, it was proved that the bioactive glass affords a controlled release of
from the glass particle surface [38], its dissemination into the agar media and its inhibitory effects upon tested pathogens growth. Similar antimicrobial findings exhibited by the glass NPs containing ZnO was reported in the literature [21]. The release of Ag from the bioactive glass of 4% concentration was further confirmed using the ion release test. Although the maximum Ag ions release value was detected in the fourth day, there was a gradual release till the eighth day. Consequently, it was proved that the bioactive glass affords a controlled release of  ions by time in the presence of moisture up to a certain limit [39].
ions by time in the presence of moisture up to a certain limit [39].
The Ag-BG 4 was the most effective antimicrobial weight percentage; therefore, it was chosen to be added to the tissue conditioner in order to prepare the composite specimens. Even though, other studies supported the antimicrobial or inhibitory potentials of tissue conditioner or denture base resin containing Ag NPs [33–36, 40]. In the present study no inhibitory or antimicrobial effects were detected in tissue conditioner containing the Ag-BG 4 NPs.
This outcome could be attributed to the fact that  is bonded inside the polymer matrix thus, limiting its release, accordingly reducing its fungicidal and antibacterial activities [41] despite the fact that the Ag peak was apparent in the elemental analysis (EDX). This is in harmony with Green et al [42] who pointed out that inhibition zone diameter is not related only to the level of antimicrobial efficiency, but similarly to the diffusion of the antimicrobial agent into the media and to the total quantity of agent available to diffuse. This finding is in agreement with a previous study conducted by Wady et al [35] who observed that AgNPs added to denture acrylic resin did not induce antifungal activity and only AgNPs aqueous solution showed antimicrobial activity against tested pathogens. The concentration and surface area of AgNPs incorporated into the polymer [43], and the characteristics of the polymer base may also affect the leaching of Ag [43, 44].
is bonded inside the polymer matrix thus, limiting its release, accordingly reducing its fungicidal and antibacterial activities [41] despite the fact that the Ag peak was apparent in the elemental analysis (EDX). This is in harmony with Green et al [42] who pointed out that inhibition zone diameter is not related only to the level of antimicrobial efficiency, but similarly to the diffusion of the antimicrobial agent into the media and to the total quantity of agent available to diffuse. This finding is in agreement with a previous study conducted by Wady et al [35] who observed that AgNPs added to denture acrylic resin did not induce antifungal activity and only AgNPs aqueous solution showed antimicrobial activity against tested pathogens. The concentration and surface area of AgNPs incorporated into the polymer [43], and the characteristics of the polymer base may also affect the leaching of Ag [43, 44].
In the current study  release test was performed to ascertain the leaching out of
release test was performed to ascertain the leaching out of  from tissue conditioner (TC) composite specimens at different intervals.
from tissue conditioner (TC) composite specimens at different intervals.  release was detected till second day (48 h) with a minimal detection limit of
release was detected till second day (48 h) with a minimal detection limit of  . Interestingly, after fourth day there was a gradual increase in the amount of
. Interestingly, after fourth day there was a gradual increase in the amount of  release reaching maximum within four weeks with obvious change in the specimens' color. Several factors may explain these findings: the presence of wet environment as deionized water [45], alteration of Ag ions into metallic Ag, and the release of silver into the surrounding media which is influenced by the dynamic dissolution of the glassy matrix in the liquid media. Consequently, glass may act as a dispenser for
release reaching maximum within four weeks with obvious change in the specimens' color. Several factors may explain these findings: the presence of wet environment as deionized water [45], alteration of Ag ions into metallic Ag, and the release of silver into the surrounding media which is influenced by the dynamic dissolution of the glassy matrix in the liquid media. Consequently, glass may act as a dispenser for  providing a precise release of
providing a precise release of  which is very important to maintain the biocidal activity for a long time [39].
which is very important to maintain the biocidal activity for a long time [39].
Another possible explanation for this remarkable increase of released  is that: the initial release of
is that: the initial release of  was from the NPs at the surface while more time was required for
was from the NPs at the surface while more time was required for  released from NPs deeply located the polymer matrix to migrate to the surface of the specimen [45]. Besides, water diffusion, allows polar groups present in the polymeric chains to be separated, and the water dipoles are then attached to those of the tissue conditioner polymer. Thus, the polymeric structure is re-assumed with water incorporation. Accordingly, a free volume is created in the polymer, which could permit molecular flexibility, ion and particle movement [44, 45]
released from NPs deeply located the polymer matrix to migrate to the surface of the specimen [45]. Besides, water diffusion, allows polar groups present in the polymeric chains to be separated, and the water dipoles are then attached to those of the tissue conditioner polymer. Thus, the polymeric structure is re-assumed with water incorporation. Accordingly, a free volume is created in the polymer, which could permit molecular flexibility, ion and particle movement [44, 45]
5. Conclusions
Within the limitations of this in vitro study the following was concluded:
- 1.Novel silver doped bioactive glass nanoparticles with different silver concentrations were developed.
- 2.The three different concentrations of silver doped bioactive glass NPs (Ag-BG 1.2, Ag-BG 3 and Ag-BG 4) showed antimicrobial activity against selected pathogens.
- 3.Silver doped bioactive glass NPs, provides gradual release of
 as a matter of time.
as a matter of time. - 4.Tissue conditioner containing silver doped bioactive glass NPs and its elutes does not possess any antimicrobial properties or cytotoxic effects, however, Ag ions release increased gradually with time attaining maximum value after four weeks
- 5.Further studies are suggested to reach the optimum percentage of Ag in bioactive glass to formulate (obtain) tissue conditioner with antimicrobial characteristics.
Acknowledgments
The authors would like to thank National Research Centre (NRC) for the financial support, Hoda El-Sayed, Department of Microbiology, Dairy Science Department (Microbiology Lab.), Food Industries and Nutrition Division, for performing and assessment of antimicrobial part.