Abstract
Improvement of biological activities of medicinal plant in combination with metal nanoparticles is highly appealing. Herein, bio-conjugated AuNP and AgNP have been synthesized at room temperature using leaf extract of Saraca asoca a medicinal plant. Phytochemicals present in aqueous leaf extract of Saraca asoca, are acting as reducing, capping and stabilizing agent. The formation of nanoparticles is confirmed and characterized by different instrumental techniques like UV-visible and IR spectroscopy, SEM, TEM and XRD analysis. Both nanoparticles are spherical, monodisperse and crystalline in nature. Phytochemicals, comprising of functional groups like aliphatic ester C–O–C, alcoholic and phenolic–OH in extract are responsible for reduction of metal precursor and functional groups like N–H or O–H are responsible for capping and stabilization of nanoparticles. Preliminary biological activities like antibacterial, anticancer and anti-diabetic activity of bio-conjugated metal nanoparticles are screened through disc diffusion methods, MTT assay and α-amylase inhibition assay. Both AuNP and AgNP show antibacterial activity (zone inhibition values 9.6 mm and 9.7 mm for E. coli and 7.7 mm and 11 mm for B. subtilis, respectively) and anti-diabetic activity (IC50 1.5 mM and 0.35 mM, respectively) whereas AgNP displays growth inhibition of prostate cancer cell line  . CAM assay confirm the biocompatibility of synthesized nanoparticles. Further, both nanoparticles show excellent catalytic activity towards the reduction of 4-nitrophenol (4-NP) using
. CAM assay confirm the biocompatibility of synthesized nanoparticles. Further, both nanoparticles show excellent catalytic activity towards the reduction of 4-nitrophenol (4-NP) using  as reducing agent even at very low concentration.
as reducing agent even at very low concentration.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 3.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Since last decades preparation of metallic nanoparticles via green synthesis approach have received a considerable interest as it offers various advantages in terms of biocompatibility, biological activity, environment friendly process etc as compared to conventional chemical process [1–4]. As a result, researchers are always trying to get uncertain exciting application using various biologically available molecules. However, still it is at its infancy as millions of living organisms are present. Moreover, development and improvement of biological activities of medicinal plant combining with metal nanoparticles is highly appealing. These bio-conjugated nanoparticles often found to display excellent anticancer, antibiotic, anti-diabetic activity and act as drug delivery [5–7]. Among different metals, silver (Ag) and gold (Au) are mostly utilized for the preparation of green synthesised metal nanoparticles and both bio-conjugated nanoparticles display good medicinal as well as catalytic activity. Bio-conjugated metal nanoparticles, especially AgNPs, possesses excellent antibacterial activity against both gram positive and gram negative bacteria. For example, AgNP prepared by using Chlorella vulgaris [8], Chamaemelum nobile [9], Cymbopogon citratus [10], Eriobotrya Japonica [11], Xanthium strumerium [12] etc have shown good antibacterial activity. In other reports, Cordia dichotoma [13]and Solidago altissima [14] mediated synthesis of AgNP have shown antibacterial activity as well as excellent photo-catalytic activity. Nanoparticles produce from Chlorella vulgaris [8] and Andrographis paniculata [15] demonstrate good anticancer activity against Hep-G2 and PC3 cancer cell lines, respectively. Govindappa et al [16] also demonstrated the antioxidant, antimicrobial, anti-inflammatory and tyrokinase inhibitory activity of AgNP prepared from Glycosmis mauritiana. Considering these aspects, in the present work Saraca asoca, (different from Saraca indica, belong to the Caesalpinioideae subfamily and legume family) plant have been used for the synthesis of metal nanoparticles. Saraca asoca is a traditional medicinal plant, commonly known as ashoka tree [17]. Phyto-constituents like carbohydrates, proteins, tannins, flavonoids, saponins, glycosides and steroids present in the leaf of Saraca asoca are responsible for various therapeutic activities like antioxidant, antibacterial, anticancer, antimenorrhagic, antioxytocic etc [18–21]. Aqueous leaf extracts of Saraca asoca have been used for the synthesis of monometallic gold and silver nanoparticles (AuNPs and AgNPs). Different instrumental techniques like UV-visible, infrared (IR) and x-ray diffraction (XRD) spectroscopies, scanning electron microscope (SEM) and transmission electron microscopy (TEM) analyses are used for the characterization of prepared nanoparticles. Preliminary screening of these nanoparticles for biological activities have been carried out by disk diffusion assay, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay and  amylase inhibition assay. Further catalytic activity has been checked using 4-nitrophenol (4-NP) reduction model.
amylase inhibition assay. Further catalytic activity has been checked using 4-nitrophenol (4-NP) reduction model.
2. Experimental
2.1. Materials
All the experiments were performed in triple distilled water and glassware were cleaned with aqua regia and washed with triple distilled water.  ,
,  , Whatman filter paper disks, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) and
, Whatman filter paper disks, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) and  amylase were purchased from Sigma Aldrich. Luria-Bertani broth was purchased from Himedia, India.
amylase were purchased from Sigma Aldrich. Luria-Bertani broth was purchased from Himedia, India.
2.2. Preparation of Saraca asoca leaf extract
Fresh leaves of Saraca asoca were collected from parking lot of Siksha O Anusandhan Deemed to be University, and thoroughly washed with triply distilled water. 10 g of Saraca asoca leaves were taken in 50 ml triply distilled water and boiled in microwave oven for 1 min. This process was repeated for 1 h interval up to 8 h and then allowed to cool at room temperature. This was then filtered and centrifuge at 14,000 rpm for 40 min. The supernatant was stored at  and used as stock solution.
and used as stock solution.
2.3. Synthesis of AuNP and AgNP
To 10 ml, 0.25 mM aqueous  solution varying amount of aqueous leaf extract of Saraca asoca (
solution varying amount of aqueous leaf extract of Saraca asoca ( ,
,  and
and  in 10 ml) were added with stirring. The formation of AuNP were confirmed by visual observation of ruby red color and the progress of the reaction was monitored by UV–vis spectrophotometer by measuring the characteristic surface plasmon resonance (SPR) band of AuNP in the wavelength range 200 nm to 800 nm. Similar synthesis procedure was followed for the preparation of AgNP. Varying amounts of leaf extract of Saraca asoca (
in 10 ml) were added with stirring. The formation of AuNP were confirmed by visual observation of ruby red color and the progress of the reaction was monitored by UV–vis spectrophotometer by measuring the characteristic surface plasmon resonance (SPR) band of AuNP in the wavelength range 200 nm to 800 nm. Similar synthesis procedure was followed for the preparation of AgNP. Varying amounts of leaf extract of Saraca asoca ( ,
,  and
and  ) were added to 10 ml, 0.25 mM aqueous
) were added to 10 ml, 0.25 mM aqueous  solution. The appearance of bright orange color indicated the formation of AgNP in solution. The bio-synthesized nanoparticle solutions were kept in refrigerator and used as stock solution for further use.
solution. The appearance of bright orange color indicated the formation of AgNP in solution. The bio-synthesized nanoparticle solutions were kept in refrigerator and used as stock solution for further use.
2.4. Characterization
Surface plasmon resonance band of biosynthesized nanoparticles were measured using Perkin Elmer Lambda 35 spectrophotometer ranging from 200 nm to 800 nm. SEM analysis was performed by using Zeiss Merlin compact Microscope, Oxford instruments by using drop crusting the samples on carbon tape, and TEM images were taken by using JEOL JEM 2100 HR-transmission electron microscope by placing the samples on copper grid. The XRD analysis was carried out using Bruker D8 Advance Diffractometer (Bruker AXS, Germany) with  radiation
radiation  placing concentrated sample on glass slide and dried at
placing concentrated sample on glass slide and dried at  for overnight. The FTIR analyses were performed using Bruker FTIR ALPHA by making KBr pellets.
for overnight. The FTIR analyses were performed using Bruker FTIR ALPHA by making KBr pellets.
2.5. Antibacterial activity study
Antibacterial activities of nanoparticles were done in E. coli MG1655 and B. subtilis 168 stains. In dried Luria-Bertani agar plate containing E.coli and B. subtilis strains, filter paper disks were placed. Then,  of AuNP, AgNP and leaves extract was spotted on paper disks and air-dried for 30 min. After that, plates were incubated at
of AuNP, AgNP and leaves extract was spotted on paper disks and air-dried for 30 min. After that, plates were incubated at  for 10 h, same concentration of ampicillin, kanamycin
for 10 h, same concentration of ampicillin, kanamycin  were taken as controls. Clear zones were noted as the inhibitory effect of nanoparticles on growing lane of culture.
were taken as controls. Clear zones were noted as the inhibitory effect of nanoparticles on growing lane of culture.
2.6. Anticancer activity study
Prostate cancer (Du145) cell line (from the ATCC) was taken for studying the anticancer activity. Cells were grown in RPMI medium (Roswell Park Memorial Institute medium) with 10% bovine serum (FBS) supplement and antibiotics. The cell viability of the green synthesized AuNP and AgNP was measured by standard MTT (3-(4, 5-dimethylthiazol-2-yl)-2-5 diphenyl tetrazolium bromide) assay by following the procedure reported earlier [22].
2.7. Anti-diabetic activity study
For study the anti-diabetic activity α-amylase inhibition assay model was used following the method as described earlier [23]. In brief, sample was mixed with 0.5 ml of  amylase solution (
amylase solution ( in 0.02 M sodium phosphate buffer, pH 6.9) and incubated at
in 0.02 M sodium phosphate buffer, pH 6.9) and incubated at  for 10 min followed by addition of 0.5 ml of starch solution (1%) and again incubated at
for 10 min followed by addition of 0.5 ml of starch solution (1%) and again incubated at  for 10 min. Finally, the reaction was terminated using 1 ml of dinitrosalicylic acid and placed in a water bath (
for 10 min. Finally, the reaction was terminated using 1 ml of dinitrosalicylic acid and placed in a water bath ( ) for 5 min. The mixture was then diluted with 10 ml of deionised water and measured the absorbance at 540 nm. During calculation absorbance intensity value of only nanoparticles should be subtracted from the test value as metal nanoparticles itself shows absorbance peak at visible region. The inhibition of
) for 5 min. The mixture was then diluted with 10 ml of deionised water and measured the absorbance at 540 nm. During calculation absorbance intensity value of only nanoparticles should be subtracted from the test value as metal nanoparticles itself shows absorbance peak at visible region. The inhibition of  amylase was calculated using the following equation:
amylase was calculated using the following equation:
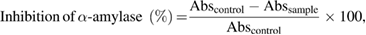
where  and
and  are the absorbance of solution without and with sample, respectively.
are the absorbance of solution without and with sample, respectively.
2.8. Toxicity study
Chick chorioallantoic membrane (CAM) assay was done to check the biocompatibility. In brief, fertilised chicken eggs were obtained from the local government poultry farm (CPDO, Bhubaneswar) at embryonic days 5. Eggs were placed horizontally and open the top of the egg shell to expose the CAM. Sterile filter paper discs were soaked with test sample (0.25 mM nanoparticles and raw extract), dried and placed on the exposed blood vessels for 5 h. Before and after 5 h of incubation, images were taken to compare the changes of blood vessels.
2.9. Catalytic activity study
Catalytic activity study of green synthesised AuNP and AgNP were performed in 4-nitrophenol (4-NP) reduction model in presence of  by following the reported method. In brief,
by following the reported method. In brief,  of nanoparticles (final concentration is 0.025 mM) suspension and
of nanoparticles (final concentration is 0.025 mM) suspension and  of
of  solution (final concentration is 10 mM) with
solution (final concentration is 10 mM) with  of water was taken in a 1 ml quartz cuvette, which was place in the cell holder of the spectrophotometer for 30 s. Then absorbance was taken immediately after adding
of water was taken in a 1 ml quartz cuvette, which was place in the cell holder of the spectrophotometer for 30 s. Then absorbance was taken immediately after adding  of 4-NP solution (final concentration is 0.1 mM) and continued the time dependent spectra measurement to monitor the reaction process.
of 4-NP solution (final concentration is 0.1 mM) and continued the time dependent spectra measurement to monitor the reaction process.
3. Results and discussion
3.1. Synthesis of metal nanoparticles
Aqueous extract of Saraca asoca leaves have been used for the synthesis of metal nanoparticles. Metal nanoparticles are successfully prepared by adding suitable ratios of metal precursors and leaf extract. It is observed that at room temperature extract acts as both reducing agent and stabilizing agent
3.2. Optical properties of metal nanoparticles
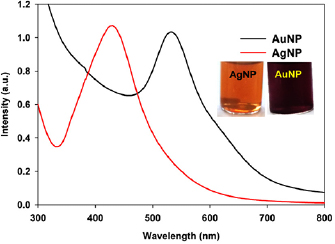
It is known that the surface plasmon resonances (SPR) property of metal nanoparticles is unique with their type of metals, size and shape. After visual observation, SPR measurement using UV-visible spectrophotometer is carried out for monitoring the nanoparticles synthesis. The observed SPR bands at 428 nm and 532 nm in the UV-visible spectra are the signature for the formation of AgNP and AuNP, respectively (figure 1). To optimize the reaction parameters, the concentration of reagents and reaction time are varied. The optimum reaction conditions are found to be  leaf extract,
leaf extract, 
 in 10 ml and 12 h reaction time for AuNP and
in 10 ml and 12 h reaction time for AuNP and  leaf extract, 0.25 mM
leaf extract, 0.25 mM  in 10 ml and reaction time 8 h for AgNP (figures S1 and S2 in supplementary (stacks.iop.org/ANSN/9/035001/mmedia)).
in 10 ml and reaction time 8 h for AgNP (figures S1 and S2 in supplementary (stacks.iop.org/ANSN/9/035001/mmedia)).
Figure 1. UV-visible spectra of the synthesised nanoparticles. [Metal precursor] = 0.25 mM, plant extract =  for AuNP and
for AuNP and  for AgNP, time = 8 h at room temperature.
for AgNP, time = 8 h at room temperature.
Download figure:
Standard image High-resolution image3.3. SEM and TEM analysis
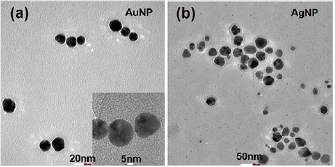
The formation of nanoparticles and their morphology, shape and size have been authenticated by SEM (figure 2) and TEM (figure 3) analyses. Both the SEM and TEM results revealed that the green synthesized AuNP and AgNP are spherical in nature with average particle size 24 nm and 36 nm, respectively, with relatively good monodispersity.
Figure 2. SEM images of (a) AuNPs and (b) AgNPs prepared by using plant extract at room temperature.
Download figure:
Standard image High-resolution imageFigure 3. TEM images of (a) AuNPs and (b) AgNPs prepared by using plant extract at room temperature.
Download figure:
Standard image High-resolution image3.4. Powder XRD analysis
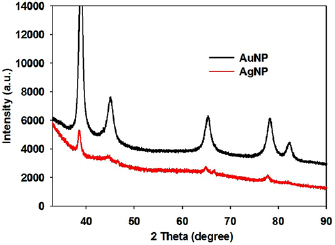
The crystalline nature and crystallite size of nanoparticles are identified by x-ray diffraction (XRD) analysis (figure 4). XRD pattern of AuNP and AgNP have shown diffraction peaks at  ,
,  ,
,  ,
,  and
and  in the
in the  range
range  which are corresponds to the (111), (200), (220), (311) and (222) planes. Both data show that the biosynthesized nanoparticles are highly crystalline in nature with a crystallite size 11–18 nm for AuNP and 15–25 nm for AgNP. The crystallite size is calculated from the full width at half maxima (FWHM) of the high-intensity diffraction peak using the Debye-Sherrer formula:
which are corresponds to the (111), (200), (220), (311) and (222) planes. Both data show that the biosynthesized nanoparticles are highly crystalline in nature with a crystallite size 11–18 nm for AuNP and 15–25 nm for AgNP. The crystallite size is calculated from the full width at half maxima (FWHM) of the high-intensity diffraction peak using the Debye-Sherrer formula:

where  is the mean grain size,
is the mean grain size,  is the x-ray wavelength for
is the x-ray wavelength for 
 ,
,  is FWHM of diffraction peak of plane (111) and
is FWHM of diffraction peak of plane (111) and  is the diffraction angle.
is the diffraction angle.
Figure 4. XRD pattern of the synthesised nanoparticles.
Download figure:
Standard image High-resolution image3.5. Infrared analysis
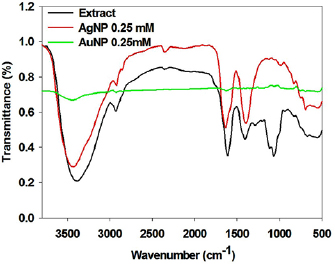
To identify the probable biomolecules responsible for reduction and stabilization of nanoparticles present in the leaf extract, Fourier transform infrared (FTIR) analysis are performed. Changes of spectral peaks between pure plant extract and biosynthesized NPs give the information about the role of possible functional groups for the synthesis of nanoparticles. The dried extract have shown intense IR bands at  ,
,  ,
,  ,
,  ,
,  ,
,  and
and  indicating the presence of (N–H or O–H), C–H, –C=O, aldehydic –C–H/C–O–H, aliphatic ester, alcoholic –C–O and –C–O–C–functional groups. The bands at 1280, 1112 and
indicating the presence of (N–H or O–H), C–H, –C=O, aldehydic –C–H/C–O–H, aliphatic ester, alcoholic –C–O and –C–O–C–functional groups. The bands at 1280, 1112 and  get vanished after the formation of nanoparticles, indicating that functional groups present in that region are responsible for reduction of metal ions and functional group corresponding to
get vanished after the formation of nanoparticles, indicating that functional groups present in that region are responsible for reduction of metal ions and functional group corresponding to  ,
,  ,
,  and
and  that are N–H or O–H, responsible for capping and stabilization of nanoparticles (figure 5).
that are N–H or O–H, responsible for capping and stabilization of nanoparticles (figure 5).
Figure 5. FTIR spectra of extract and synthesised nanoparticles.
Download figure:
Standard image High-resolution image3.6. Biological activity
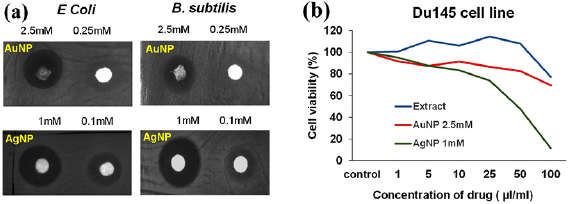
Antibacterial activity of AgNP is well known but AuNP usually does not show antibacterial activity. Recent report discloses that sub-nanometer dimension AuNP displays good antibacterial activity [24]. The preliminary screening for antibacterial potency of bio-synthesized AuNP and AgNP are done by disc diffusion assay using gram negative E. coli and gram positive B. subtilis strains. 1 mM of AgNP displays very good antibacterial activity with zone of inhibition 9.7 mm and 11 mm whereas 2.5 mM of AuNP shows antibacterial activities at high concentration with zone of inhibition 9.6 mm and 7.7 mm for E. coli and B. subtilis respectively (figure 6(a) and table S1). These results are comparable with standard antibiotics kanamycin and ampicillin (figure S3). Previous literature also reports the good antibacterial activity of as-synthesized AgNP against E. Coli and B. subtilis with zone of inhibition 15.1 nm and 14.3 nm using concentration  and
and  , respectively [9]. Screening of anticancer activity by MTT assay shows that AgNP significantly inhibits the growth of prostate cancer cell line DU145 (p53 mutant) with IC50 of
, respectively [9]. Screening of anticancer activity by MTT assay shows that AgNP significantly inhibits the growth of prostate cancer cell line DU145 (p53 mutant) with IC50 of  whereas AuNP is inactive (figure 6(b) and table S1). Prasannaraj et al also reports the anticancer activity of green synthesized AgNP on prostate cancer cell line PC3 where p53 is null. This indicates the anticancer activity of AgNP may be p53 independent [15]. The antidiabetic activities of bio-synthesized nanoparticles are also tested by
whereas AuNP is inactive (figure 6(b) and table S1). Prasannaraj et al also reports the anticancer activity of green synthesized AgNP on prostate cancer cell line PC3 where p53 is null. This indicates the anticancer activity of AgNP may be p53 independent [15]. The antidiabetic activities of bio-synthesized nanoparticles are also tested by  amylase inhibitory assay. Both nanoparticles, inhibit
amylase inhibitory assay. Both nanoparticles, inhibit  amylase activity with IC50 value of
amylase activity with IC50 value of  and 0.35 mM for AuNP and AgNP, respectively (table S1 and figure S4). The in vitro toxicity study of nanoparticles are done by chicken embryo assay (CAM assay), which confirm the biocompatibility of green synthesized nanoparticles (figure S5).
and 0.35 mM for AuNP and AgNP, respectively (table S1 and figure S4). The in vitro toxicity study of nanoparticles are done by chicken embryo assay (CAM assay), which confirm the biocompatibility of green synthesized nanoparticles (figure S5).
Figure 6. Screening of (a) antibacterial activity on E. coli and B. subtilis strains, and (b) anticancer activity on prostate cancer cell line (DU145) using synthesized nanoparticles.
Download figure:
Standard image High-resolution image3.7. Catalytic activity
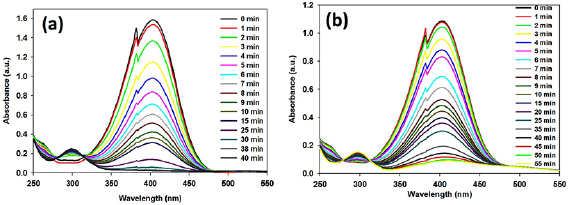
It is known that nitro-aromatic compounds are the main organic hazard in environment, having mutagenic or carcinogenic activities. Therefore, to minimize the toxic nitro-phenol, conversion of nitro group to amine group is crucial. Metal nanoparticles acts as catalyst for this conversion. Here synthesized NPs exhibited excellent catalytic performance toward the reduction of 4-nitrophenol (4-NP) by  in a water-based system (figure 7).
in a water-based system (figure 7).
Figure 7. Catalytic activity of (a) AuNP, and (b) AgNP on 4-nitrophenol reduction model using sodium borohydride.
Download figure:
Standard image High-resolution image4. Conclusion
In conclusion, the present study demonstrates successful synthesis of monometallic AuNP and AgNP nanoparticles using leaf extract of Saraca asoca. The UV-visible spectra, SEM and TEM data suggest that both AuNP and AgNP are monodispersed and spherical in nature with a particles size 23 nm and 29 nm, respectively. XRD analysis suggests the crystalline nature of the nanoparticles. The FTIR study suggests the conjugation of biomolecules on the surface of nanoparticles. Both AuNP and AgNP display antibacterial and anti-diabetic activity, however, AgNP only shows anticancer activity. The present study demonstrates such an example of antibacterial activity of AuNP which is normally at the rear (only shows when it is very small in diameter). Catalytic activities of synthesised nanoparticles have shown that AuNPs are more active than AgNPs. Thus, we believe that the present work is an important addition to the research of scientific community who are working on the green synthesis of metal nanoparticles and development of nanomedicine.
Acknowledgment
N P would like to acknowledge Siksha 'O' Anusandhan Deemed to be University for her doctoral fellowship and IIT Bhubaneswar for laboratory facilities.