Abstract
Selenium and silver nanoparticles (NPs) were synthesized using Spermacoce hispida aqueous leaf extract (Sh-ALE). The optimum condition required for the synthesis of Sh-SeNPs was found to be 30 mM selenious acid solution to Sh-ALE at the ratio of 4:46, pH 9, incubated at 40 °C for 10 min. On the other hand, for Sh-AgNPs the optimum condition was found to be 1 mM AgNO3 to the Sh-ALE solution at the ratio of 4:46, pH 8, incubated at 40 °C for 10 min. SEM analysis revealed that both the Sh-AgNPs and Sh-SeNPs are predominantly rod-shaped. Sh-SeNPs and Sh-AgNPs were found to possess concentration-dependent antioxidant activity. However, Sh-SeNPs showed potent anti-inflammatory property, antibacterial property and anticancer activity against human cervical cancer cell in comparison to Sh-AgNPs. Phytochemical analysis, FTIR and GC-MS analysis showed that various flavonoids, saponins and phenolic compounds present in Sh-ALE catalysed the formation of NPs. Also, GC-MS analysis revealed that Sh-SeNPs are capped by synaptogenin B and derivatives of apigenin, quinoline and quinazoline. The advantage of attachment of such phytoconstituents on Sh-SeNPs for its potent biological activity in comparison to Sh-AgNPs is evident in in vitro conditions.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 3.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Nanoparticles (NPs) are gaining tremendous attention in the biomedical field. The chemical methods used in the synthesis of NPs are considered disadvantageous due to requirement of special equipment, high expense and environmental hazard. Hence, green synthesis of NPs using microorganisms [1], plants [2] and secondary metabolites extracted from natural sources has gained importance. In particular, plant extracts which are enriched in enzymes (hydrogenases, reductases) and phytochemicals (viz. terpenoids, flavonoids, phenols and dihydric phenols) are considered advantageous in NPs preparation [3]. These components act as reducing agents and stabilizers in the conversion of a metal salt into NPs. Silver NPs (AgNPs) and selenium NPs (SeNPs) synthesized using various plant extracts are shown to exhibit various biological activities such as antimicrobial, antioxidant, anti-inflammatory, inhibition of enzymes, anti-diabetics and anti-cancer activity [4–8]. Hence, the need for synthesis of NPs using various other medicinal plants towards biological application is in demand. Keeping these facts in view in the present study, AgNPs and SeNPs were synthesized using an aqueous leaf extract of Spermacoce hispida (S. hispida) (Sh-ALE). S. hispida belonging to Rubiaceae family are widely used in the treatment of diabetes and cardiovascular diseases. S. hispida are rich in secondary metabolites like flavonoids, tannins, saponins, terpenoids, steroids, lignin and phenols which are involved in the medicinal property of this plant. In specific, various literatures show that S. hispida is an important source of pharmacologically important phytoconstituents such as borreline, β-sitosterol, ursolic acid and isorhmnatin [9]. The plant has also been widely studied for their various pharmacological activities such as antidiabetic, antihypertensive, antioxidant, analgesic, anticancer, hepatoprotective and antifungal activity. The AgNPs and SeNPs synthesized using medicinally important Sh-ALE (Sh-AgNPs and Sh-SeNPs) has been characterized using a UV–visible spectrophotometer, scanning electron microscopy (SEM), Fourier transform infrared spectroscopy (FTIR), x-ray diffraction (XRD), energy dispersive x-ray (EDX) spectroscopy and BET (Brunauer, Emmett and Teller) analysis. Further, the biological property of the Sh-AgNPs and Sh-SeNPs such as antioxidant, antibacterial, anti-inflammatory and anticancer activity in in vitro are analyzed. The phytoconstituents: (i) present in Sh-ALE and (ii) bound on the Sh-SeNPs were determined using gas chromatography mass spectrophotometer (GC-MS) analysis in order to understand the involvement of biogenic nature of synthesized NPs for its biological activity. The results obtained in the present study shows that the biological activity of Sh-SeNPs is higher than that of Sh-AgNPs.
2. Materials and methods
2.1. Plant material
Fresh leaves of S. hispida were collected from Bommidi, Dharmapuri, Tamil Nadu, India. The voucher specimen was identified on 20-02-2016 in ABS Botanical Conservation, Research and Training Centre, Kaaripatti-636106, Salem (Dt) Tamil Nadu, India (Reference number AUT/ PUS/ 092).
2.2. Chemicals and cell line
All the reagents were of analytical grade. Silver nitrate, selenious acid and 2,2-diphenyl-1-picrylhydrazyl (DPPH) were purchased from Loba Chemie, India. 2,2'-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) (ABTS) was purchased from Sigma, USA. Quercetin was purchased from Himedia, India. All the solvents used in the study were purchased from Merck, India. All aqueous solutions were prepared with distilled and de-ionized water. The human cervical cancer cell lines (HeLa) was obtained from National Centre for Cell Science, Pune, India.
2.3. Preparation of aqueous extract of S. hispida leaves
The fresh leaves of S. hispida were washed with distilled water to remove the dust particles and chopped into small pieces. The Sh-ALE was prepared by boiling 5 g of chopped material in 100 ml of distilled water for 5 h. The extract obtained was filtered using Whatman no. 1 filter paper. The supernatant was collected and stored in an airtight container at 4 °C until further use.
2.4. Optimization of various parameters for synthesis of NPs using Sh-ALE
Various factors like pH, temperature, incubation time and ratio of AgNO3 or selenious acid to Sh-ALE were optimized for the synthesis of Sh-SeNPs or Sh-AgNPs. The experiments were carried out in 50 ml Erlenmeyer flask containing various ratios (0.5:49.5, 2:48, 4:46 or 6:44) of AgNO3 (1 mM) or selenious acid (30 mM) to Sh-ALE. The pH of the mixture was adjusted using sodium hydroxide (0.1 N) or hydrochloric acid (0.1 N) either to 6, 7, 8, 9 or 10. The reaction was carried out at different temperatures (4 °C, 20 °C, 40 °C or 60 °C) for 15 min. The aqueous solution of AgNO3 or selenious acid was maintained as control throughout the experiment. The effect of these parameters on the synthesis of Sh-AgNPs and Sh-SeNPs was monitored using UV–vis spectrophotometer (Shimadzu UV 1800 UV/Vis spectrometer) by scanning between 300 and 600 nm. At the end of the incubation period, the NPs produced were harvested by centrifugation at 5000 rpm for 30 min [10]. The Sh-AgNPs and Sh-SeNPs obtained were washed with distilled water for three times to remove excess silver or selenium ions and other fine particles. The NPs were stored at 4 °C until further use.
2.5. Structural characterization
The morphology and size of the NPs were investigated by using SEM (TESCAN, Czech Republic) provided with Vega TC Software. The chemical property of NPs is examined by EDX spectroscopy. The total surface area of NPs was determined by the method of BET using metrometrics ASAP 2010 surface area analyzer (USA). The XRD analysis of dried powder of NPs was carried out on an XRD instrument (Bruker, AXS system, D8, Germany) with the scanning range between 20° and 80°. FTIR analysis (Perkin Elmer, USA) was used to determine the surface functional groups of the NPs by scanning through wave length 4000–500 cm−1.
2.6. Qualitative and quantitative analysis of phytoconstituents
The Sh-ALE, Sh-AgNPs and Sh-SeNPs were screened for the presence of various phytochemicals, like flavonoids, alkaloids, tannins, saponins, phenols and glycosides using standard color tests [11]. The total flavonoid content (TFC), total phenolic content (TPC) and total saponin content (TSC) were determined spectrophotometrically using aluminum chloride, Folin–Ciocalteau reagent and vanillin, respectively [12, 13]. Quercetin and diosgenin were used as standard. The TPC, TFC and TSC were expressed in terms of standard equivalent (µg g−1 of starting plant material or NPs).
2.7. In vitro antioxidant activity of Sh-AgNPs and Sh-SeNPs
Various antioxidant activities such as scavenging activity of DPPH, hydrogen peroxide (H2O2) and ABTS as well as ferric reducing antioxidant power (FRAP) assay were carried out as described earlier [14]. For different radical scavenging activities, 20–100 µg ml−1 concentration of NPs was used. For FRAP assay, 10–50 µg ml−1 concentrations of NPs were used.
2.8. Antibacterial activity
The antibacterial activity of the Sh-SeNPs and Sh-AgNPs was evaluated against gram positive bacteria, like Staphylococcus aureus, Enterococcus and gram negative bacteria, like Klebsiella pneumoniae, Escherichia coli using disc diffusion method. The samples were inoculated (108 CFU ml−1) in petri dishes with nutrient agar medium and then paper disks of 5 mm in diameter were laid on the inoculated test organism, which was instilled with NPs at different concentrations (25 and 50 µg ml−1). The plates were incubated at 37 °C for 24 h. Antimicrobial activity was determined by measuring the zone of inhibition (mm) around the disk. The mean reported for each type of NP and each microbial strain was tested in triplicate.
2.9. Anticancer activity
The anti-cervical cancer activity of Sh-AgNPs and Sh-SeNPs was assessed against HeLa cell lines. Cell line was incubated with various concentration of NPs for 24 h and the cell viability was determined using 3-(4,5-dimethylthiazol-2-yl)2,5-diphenyltetrazolium bromide (MTT) assay. Data were expressed as percent cell viability relative to control cultures [15].
2.10. Anti-inflammatory activity of NPs
The anti-inflammatory activity of the synthesized NPs as a measure of protein denaturation inhibition was studied through in vitro assay [16]. Bovine serum albumin (BSA) solution (1%) was incubated at room temperature for 30 min with or without different concentrations (10–50 µg ml−1) of NPs. The pH of the solution was adjusted to 2 using drop-wise addition of concentration HCl. After incubation, the mixture was heated at 72 °C for 30 min. Finally all tubes were cooled for 10 min and the turbidity was measured at a wavelength of 660 nm. Diclofenac was used as standard.
2.11. GC-MS analysis of Sh-ALE and Sh-SeNPs
Sh-SeNPs suspension was prepared by dissolving 10 mg of freeze-dried formulation powder in 5 ml of phosphate buffer saline (0.1 M PBS, pH 7.4) containing 0.1% v/v of Tween 80 (to maintain a sink condition) as described earlier [17]. The Sh-SeNPs suspension was kept in a shaker incubator set at 120 rpm and 37 °C for 24 h. The suspension was taken out from shaker and centrifuged at 10 000 rpm for 10 min. The supernatant was lyophilized and dissolved in 200 µl of PBS. The phytoconstituents present in supernatant was determined by GC-MS analysis performed using the equipment Thermo GC-Trace Ultra Version: 5.0, Thermo MS DSQ II. The identification of components was based on Willey and NIST libraries as well as comparison of their retention indices.
3. Results and discussion
3.1. Effect of various parameters on the synthesis of NPs using Sh-ALE
3.1.1. Metal ion to plant extract ratio.
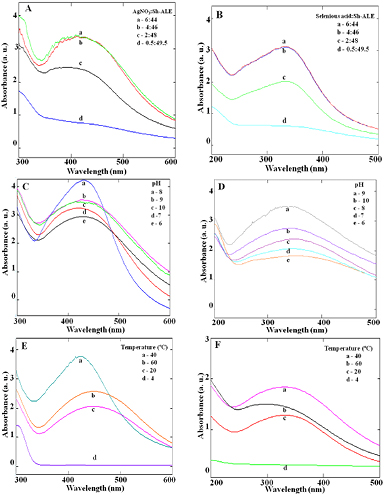
The UV–vis spectroscopic analysis of Sh-AgNPs and Sh-SeNPs synthesized using different concentration ratios of Sh-ALE to the metal salt solution are shown in figures 1(A) and (B), respectively. The absorption peak at 435 nm for Sh-AgNPs gradually increased as the concentration of Sh-ALE was increased in the AgNO3 solution (1 mM). The optimum ratio of 1 mM AgNO3 solution to Sh-ALE for efficient synthesis of Sh-AgNPs was found to be 4:46. Similarly, for Sh-SeNPs synthesis, the maximum absorption peak at 350 nm was found at the ratio of 4:46 of 30 mM selenious acid solution to Sh-ALE. Optimum plant extract to metal salt solution is required for the symmetrical NPs formation [18]. The increase in the volume of Sh-ALE might increase the concentration of reducing agents present in the plant extracts which would enhance the rate of reduction of metal ions.
Figure 1. Effect of various parameters on the synthesis of silver and selenium NPs using Sh-ALE. Representative graph showing the formation of AgNPs (A), (C) and (E) and SeNPs (B), (D) and (F) using Sh-ALE as a measure of absorbance at 435 nm and 350 nm, respectively, under various conditions like metal ion to plant extract ratio (A) and (B), pH (C) and (D) and temperature (E) and (F).
Download figure:
Standard image High-resolution image3.1.2. pH.
The 1 mM AgNO3 solution to Sh-ALE and 30 mM selenious acid solution to Sh-ALE at the ratio of 4:46 was maintained at different pH for 10 min to study the efficient formation of respective NPs. The optimum pH required for the efficient synthesis of Sh-AgNPs and Sh-SeNPs was found to be 8 and 9, respectively (figures 1(C) and (D)). Acidic pH (1–4) condition suppressed the formation of NPs (data not shown), but the basic pH condition enhanced the formation of NPs. Alkaline condition is essential for the efficient NPs formation while acidic pH had resulted in the aggregation and formation of large sized NPs. The phytoconstituents are likely to be inactive under extremely acidic conditions thereby failed to synthesis NPs. Also, NPs are reported to show maximum stability when synthesized and stored at the alkaline conditions [19].
3.1.3. Incubation temperature.
The optimum temperature for the formation of NPs was found to be 40 °C for both the NPs (figures 1(E) and (F)). At low temperature (4 °C), there was no NPs formation. However, as the incubation temperature was increased to 40 °C, Sh-AgNPs formation was associated with the development of sharp absorbance peak, which even shifted towards lower wavelength region indicating the smaller size of the NPs. These results are in good agreement with previous study which showed higher incubation temperature associated with small-sized NPs formation [19].
Overall, the optimum condition required for the synthesis of Sh-AgNPs was found to be 1 mM AgNO3 solution to Sh-ALE at the ratio of 4:46, pH 8, incubated at 40 °C for 10 min (figure 2(A)). On the other hand, for Sh-SeNPs synthesis the optimum condition was found to be 30 mM selenious acid solution to Sh-ALE at the ratio of 4:46, pH 9, incubated at 40 °C for 10 min (figure 2(B)). The synthesis of both the NPs was observed within 10 min and the formation was stabilized after that time period (data not shown). However, only a few studies have reported the rapid formation of NPs using plant extracts within 7 to 15 min [20–22]. The results obtained in the present study using Sh-ALE are equally fast (within 10 min) and efficient in the synthesis of NPs. All the plant extracts may not produce two or more different types of NPs except for few as reported earlier [20, 23] in such scenario, the advantage of rapid production of both Sh-AgNPs and Sh-SeNPs in a single step, facile, low-cost involvement, eco-friendly and safe procedure using Sh-ALE cannot be ignored.
Figure 2. Representative photographs showing the green synthesis of (A) Sh-AgNPs and (B) Sh-SeNPs.
Download figure:
Standard image High-resolution image3.2. Physiochemical characterization of NPs
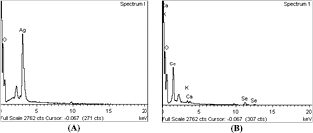
Both the Sh-AgNPs and Sh-SeNPs are well dispersed and predominantly rod-shaped, while some of the NPs were found to be irregularly shaped (figures 3(A) and (B)). The NPs are quite uniformly distributed, which indicates the stabilization of NPs by phytochemicals present in the Sh-ALE as capping agents. The average size of Sh-AgNPs and Sh-SeNPs was found to be 470 ± 20 nm and 120 ± 15 nm, respectively. The XRD pattern of the Sh-AgNPs showed a diffraction peak at 38 corresponding to Sh-AgNPs (figure 4(A)). According to JCPDS standards of XRD, the most intense peaks observed for Sh-AgNPs are related to 2θ values of 32°, 38.121°, 44.307°, 55°, 64.456° and 78.414° which corresponds to (1 2 2), (1 1 1), (2 0 0), (1 4 2), (2 2 0) and (3 1 1) planes of silver metal based on face centered cubic structure. The sharp band indicates the crystalline nature of Sh-AgNPs stabilized with the reducing agents [24]. Also, Sh-SeNPs exhibited a standard XRD pattern at 23°, 30° and 43° indexed to (1 0 0), (1 0 1) and (1 1 0) which confirms the nanoscale character of Sh-SeNPs and it is similar to SeNPs originated from all different sources [25]. The presence of broad diffraction peaks at lower angles confirms the amorphous nature of the Sh-SeNPs [26]. The appearance of other peaks might be due to conjugation of organic compounds on the surface of both the NPs, which are similar to the results obtained earlier [27, 28]. The BET analysis shows the surface area of Sh-AgNPs and Sh-SeNPs to be 19.23 and 39 m2 g−1, respectively. The EDX showed signals for the presence of silver and selenium atoms, respectively, in the Sh-AgNPs and Sh-SeNPs (figures 5(A) and (B)). The appearance of peaks of other elements such as Ca and K might be due to their presence in the plant extracts. In addition, the identification of element O might be due to organic materials bound on the NPs.
Figure 3. Representative SEM images of (A) Sh-AgNPs and (B) Sh-SeNPs.
Download figure:
Standard image High-resolution imageFigure 4. XRD spectra of (A) Sh-AgNPs and (B) Sh-SeNPs.
Download figure:
Standard image High-resolution imageFigure 5. EDX analysis illustrating the formation of (A) Sh-AgNPs and (B) Sh-SeNPs.
Download figure:
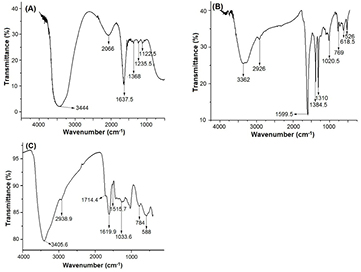
Standard image High-resolution imageFTIR provides the information about functional groups present in the synthesized NPs due to conjugation of different phytochemicals of Sh-ALE which would act simultaneously as reducing, stabilizing and capping agent. Figure 6(A) shows the FTIR absorbance band of Sh-ALE at 3444 cm−1, 1637 cm−1, 1368 cm−1 and 1122 cm−1 which are associated with O–H, C=C, C–H, and C–N stretching in Sh-ALE. In the synthesized Sh-AgNPs, absorbance bands were observed at 3362 cm−1, 2926 cm−1, 1599 cm−1, 1384 cm−1, 1310 cm−1, 1020 cm−1 and 768–526 cm−1 which correspond to O–H group, C–H, N–H bend stretching (charged amines), C–H stretching of alkanes, C–N stretching of aromatic amines, C–N stretching of aliphatic amines and vibration of metal–N or metal–O, respectively (figure 6(B)). FTIR spectrum of Sh-SeNPs (figure 6(C)) showed absorption bands at 3405 cm−1, 2938 cm−1, 1714 cm−1, 1619 cm−1, 1515 cm−1 1033 cm−1 and 786-588 cm−1 characteristic of O–H group, C–H stretching, C=O stretch, C=C, N–O asymmetric stretch, C–N stretching of aliphatic amines and metal–N/O vibration, respectively. These FTIR spectra confirm the presence of an aliphatic amine, polyol groups and alcohols in the Sh-ALE which may act as reducing agents for the synthesis of NPs. The band shift at 3362 cm−1 for Sh-AgNPs and 3405 cm−1 for Sh-SeNPs indicated the -OH group of polyols taken part in the bioreduction process. In addition, band shift at 1619 cm−1 for Sh-SeNPs indicates the involvement of saponins in NPs formation. The existence of −OH, −C=O, C–H and C=C bands in absorption peak of FTIR spectrum was characteristic of saponin present in the Sh-SeNPs. The presence of bands at 750–500 cm−1 in Sh-AgNPs and Sh-SeNPs confirms that some of the bioorganic compounds from Sh-ALE formed a strong coating/capping on the NPs.
Figure 6. FTIR spectra of (A) Sh-ALE, (B) Sh-AgNPs and (C) Sh-SeNPs.
Download figure:
Standard image High-resolution image3.3. Phytochemical screening of Sh ALE, Sh-AgNPs and Sh-SeNPs
The qualitative analysis of phytochemicals revealed the presence of flavonoids, tannins, saponins, terpenoids, steroids, lignin and phenols in the Sh-ALE. However, in Sh-AgNPs, only phenols and flavonoids are present. Similarly, Sh-SeNPs contains flavonoids, phenols and saponins. The quantitative analysis determined the concentration of TFC to be 35 ± 5.14 mg g−1, 22.8 ± 0.11 mg g−1 and 28 ± 0.18 mg g−1 in the starting plant material, Sh-AgNPs and Sh-SeNPs, respectively. The concentration of TPC was found to be 48.5 ± 4.08 mg g−1, 32.4 ± 0.05 and 36 ± 0.09 mg g−1 in the starting plant material, Sh-AgNPs and Sh-SeNPs, respectively. The concentration of TSC was found to be 29 ± 3.14 and 21 ± 0.34 mg g−1 in the starting plant material and Sh-SeNPs, respectively. The phytochemical analysis reproduced the results from FTIR spectrum obtained for Sh-AgNPs and Sh-SeNPs. Our results are consistent with the phytochemical composition of S. hispida plant as already reported which are known for its rich source of flavonoids. In accordance with our result, the flavonoids, phenols and saponins from various plant extracts are found to be involved in the NPs synthesis [29, 30]. Biofunctionalization of NPs using organic compounds present in plant extracts might increase it's biological activity. Hence, we tested the antioxidant, antibacterial, anti-inflammatory and anticancer activity of surface-biofunctionalized Sh-AgNPs and Sh-SeNPs.
3.4. Biological application of Sh-AgNPs and Sh-SeNPs: antioxidant, antibacterial and anticancer activity
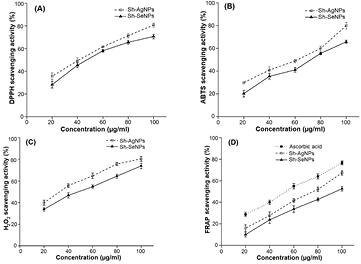
Both Sh-AgNPs and Sh-SeNPs were found to possess concentration-dependent scavenging activity on tested free radicals (figure 7). Percentage inhibition increased gradually to its maximum level with higher concentrations of NPs. In DPPH assay, the half maximal inhibitory concentration (IC50) values were found to be 42 and 46 µg ml−1 for Sh-AgNPs and Sh-SeNPs, respectively. In ABTS assay, the IC50 values were found to be 61 and 72 µg ml−1 for Sh-AgNPs and Sh-SeNPs, respectively. In the scavenging activity of H2O2, the IC50 values of Sh-AgNPs and Sh-SeNPs were found to be 33 and 48 µg ml−1, respectively. Sh-AgNPs and Sh-SeNPs showed ferric ion reducing activity in comparison to ascorbic acid. The antioxidant effect of Sh-AgNPs and Sh-SeNPs might be due to various mechanisms like hydrogen donating (DPPH and ABTS stabilizing), electron donating (ferric ion reduction) as well as scavenging of the oxidants (H2O2 clearance).
Figure 7. Antioxidant activities of Sh-AgNPs and Sh-SeNPs. (A) DPPH radical scavenging activity, (B) ABTS scavenging activity, (C) H2O2 scavenging activity and (D) FRAP activity of Sh-AgNPs and Sh-SeNPs at various concentrations are shown. Both Sh-AgNPs and Sh-SeNPs showed potent antioxidant activity. FRAP activity of Sh-AgNPs and Sh-SeNPs is higher in comparison to standard ascorbic acid. Results are expressed as mean ± SD (n = 6).
Download figure:
Standard image High-resolution imageThe antibacterial activities of different NPs as a measure of zone of inhibition was shown in table 1. It is surprising that Sh-AgNPs did not show any effect on the growth of the bacterial strain tested. However, the Sh-SeNPs showed significant inhibition against the growth of S. aureus and E. coli. The result from the present study clearly indicates that bioactive compounds on the surface of the Sh-SeNPs likely contributed to their antibacterial activities and enhanced the effect of inorganic metal part. Lack of such phytoconstituents as capping agent might be reason for the absence of antibacterial activity by Sh-AgNPs. Previous study has also shown that biogenic SeNPs was effective against bacteria and fungus in comparison to synthetically synthesized SeNPs [31]. Understanding the molecular mechanism involved in the antibacterial property of SeNPs is in its infancy, however, in some studies, bacteriostatic property [32] and reactive oxygen species (ROS) generation [33] by SeNPs are identified. Nevertheless, further detailed study is required to fully elucidate the mechanism of antibacterial effect of Sh-SeNPs.
Table 1. Antibacterial activity of Sh- SeNPs and Sh- AgNPs against clinical pathogens.
| Samples | Concentration in disc (µg ml−1) | Zone of inhibition (mm) | |||
|---|---|---|---|---|---|
| Enterococci | S. aureus | K. pneumoniae | E. coli | ||
| Sh- SeNPs | 25 | — | 14 | — | 5 |
| 50 | — | 26 | — | 11 | |
| Sh- AgNPs | 25 | — | — | — | — |
| 50 | — | — | — | — | |
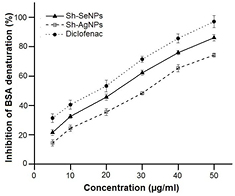
The in vitro anti-inflammatory activity or the ability of different NPs to inhibit protein denaturation was studied through inhibiting heat-induced albumin denaturation (figure 8). The IC50 value against protein denaturation of Sh-SeNPs, Sh-AgNPs and diclofenac was found to be 22.9, 31.1 and 17.8 µg ml−1, respectively. Denaturation of tissue proteins results in its loss of function and causes inflammation. Sh-SeNPs that showed efficient prevention of BSA denaturation can be further analysed for its anti-inflammatory activity in vivo. The in vitro anti-inflammatory activity of Sh-SeNPs is significantly higher than AgNPs and zinc oxide NPs synthesized using aqueous extracts of two mangrove plant species, Heritiera fomes and Sonneratia apetala [34].
Figure 8. Effect of Sh-AgNPs and Sh-SeNPs on denaturation of BSA. The effect of various concentrations of Sh-AgNPs, Sh-SeNPs and diclofenac (standard drug) against heat-induced denaturation of BSA are shown. The inhibition of BSA denaturation are expressed in % variation in comparison to control (without any test compound treatment) as mean ± SD (n = 6).
Download figure:
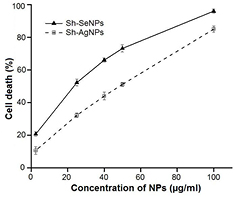
Standard image High-resolution imageCervical cancer is one of the most common gynecological malignancies and leading cause of cancer mortality among women in developing countries [35]. Hence, identifying the potential of Sh-AgNPs and Sh-SeNPs against cervical cancer will be useful for its application as an anticancer agent. Treatment with Sh-AgNPs and Sh-SeNPs showed a dose-dependent decrease in HeLa cell line viability (figure 9). The IC50 values of the Sh-AgNPs and Sh-SeNPs for inducing cell death was found to be 48.98 µg ml−1 and 22.9 µg ml−1, respectively. Surface-functionalized NPs (coated with organic compounds) like Sh-AgNPs and Sh-SeNPs are advantageous over non-functionalized (uncoated) NPs in inducing cancer cell death. The surface coating of NPs prevents agglomeration and increase the individual particle distribution. Also, functionalized NPs would have increased surface area which in turn would enhance the accessibility of NPs to membrane-bound organelles [36]. At first glance, it may be considered the irony that Sh-AgNPs and Sh-SeNPs with efficient antioxidant activity to cause cancer cell death. Although it is intensely debated regarding the use of antioxidants in cancer therapy [37] the efficacy of natural antioxidants in inducing apoptosis of cancer cells cannot be ignored [38]. Similarly, Sh-AgNPs and Sh-SeNPs has antioxidant property, it may be converted into pro-oxidant due to presence of high concentration of copper ions and might result in DNA fragmentation in the cancer cells. Also, redox reaction between metal ions and NPs might generate a variety of ROS [39]. In addition, modulation of a functional property of proteins, decreasing the intracellular glutathione and hindering with mitochondrial function by NPs might result in ROS generation as a secondary effect [40]. Overall, the cancerous environment has led the Sh-AgNPs and Sh-SeNPs to gravitate towards the pro-oxidant state. The antioxidant state of Sh-AgNPs and Sh-SeNPs has been overwhelmed by its pro-oxidant state and resulted in cancer cell death.
Figure 9. Effect of Sh-AgNPs and Sh-SeNPs on cell viability. HeLa cells were treated with various concentrations of Sh-AgNPs or Sh-SeNPs for 24 h at 37 °C and cell viability was measured as described under materials and methods. The viability of cells are expressed in % variation in comparison to control cells (without Sh-AgNPs or Sh-SeNPs treatment) as mean ± SD (n = 6).
Download figure:
Standard image High-resolution image3.5. Identification of phytoconstituents attached on the surface of Sh-SeNPs
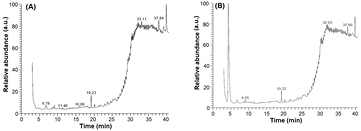
The Sh-SeNPs showed better biological activity in comparison to Sh-AgNPs. The biological activity of NPs is concentration-size-surface chemistry dependent hence, the role of phytoconstituents from Sh-ALE functionalized on the surface of Sh-SeNPs in its antibacterial, anti-inflammatory and anticancer effect is important. To understand the importance of biofunctionalization of Sh-SeNPs, the phytoconstituents present in Sh-ALE and Sh-SeNPs were analysed using GC-MS. The GC-MS chromatographic spectrum is shown in figure 10. The detail of major phytoconstituents present in Sh-ALE is given in table 2. The detection of different kinds of flavones, aglycone of saponin and phenolic compounds in Sh-ALE is in agreement with the FTIR and phytochemical screening analysis. It is apparent that these compounds present in Sh-ALE acted as reducing agents in the synthesis of NPs. In addition, some of these compounds like, 5,7-dihydroxy-6-methoxy-2-(3,5-dihydroxy-4-methoxyphenyl)-4H-1-benzopyran-4-one (a derivative of apigenin), 4-N-(4'-chlorophenyl)amino-6,7-dimethoxyquinazoline, 4-(4-chlorophenyl)-1-phenylisoquinoline, 1-(4-chlorophenyl)-4-phenylisoquinoline and synaptogenin B are detected in the supernatant of Sh-SeNPs. Apigenin is known for various biological activities, like, anticancer, prevention of pulmonary fibrosis, arthritis, type 2 diabetes, pulmonary fibrosis, asthma, hypertension and cardiac hypertrophy [41]. The skeleton structures of quinoline and quinazoline are important pharmacophore. A number of quinoline and its derivatives have antimicrobial, antitumor, antifungal, hypotensive, anti HIV, analgesics and anti-inflammatory activities [42]. Quinazolines possess antibacterial, antifungal, anticonvulsant, anti-inflammatory, anti-HIV, anticancer and analgesic activities [43]. Synaptogenin B is aglycone of synaptosides (triterpene glycosides) reported for its cytotoxic activity against HeLa cell line [44]. The important presumption from the present study is that although two different metal NPs was synthesized efficiently using Sh-ALE, the attachment of bioactive organic compound on the Sh-SeNPs and their release under in vitro conditions (cancer cells and bacterial cells) has indubitably differentiated the biological functional potential of Sh-SeNPs in comparison with Sh-AgNPs. In addition, it is advantageous to use the aqueous extract of medicinally important plant like S. hispida, which had resulted in the attachment of various pharmacologically important phytoconstituents on the surface of NPs and synergistically impose various biological applications.
Table 2. Phytochemical composition of Sh-ALE.
| Systemic name | Molecular formula | Molecular weight |
|---|---|---|
| 5,7-Dihydroxy-6-methoxy-2(3,5-dihydroxy-4-methoxy phenyl)-4H-1-be nzopyran-4-one | C17H14O8 | 346 |
| 1-(4-Chlorophenyl)-4-phenylisoquinoline | C21H14ClN | 315 |
| 2,6,9-Tribromo-4-methoxy-furo[3,2-g] coumarin | C12H5Br3O4 | 450 |
| 4,4'-Isopropylidene-BIS-(2-cyclohexylphenol) | C27H36O2 | 392 |
| 4à,14à,24-trimethyl-9(11)-cholesten-3á-yl acetate | C32H54O2 | 470 |
| 4-N-(4'-Chlorophenyl)amino-6,7 dimethoxy quinazoline | C16H14ClN3O2 | 315 |
| 4-(Methylthio) catechol | C7H8O2S | 156 |
| Phthalic acid, pentadecyl 2,4,4-trimethylpentyl ester | C31H52O4 | 488 |
| 4-(4-Chlorophenyl)-1-phenylisoquinoline | C21H14ClN | 315 |
| Dibenzo[f,h]cinnolin-3(2H)-one | C16H10N2O | 246 |
| Synaptogenin B | C30H46O4 | 470 |
| 3á,11à,15à-trihydroxycycloart-24- en-26-oic acid | C30H48O5 | 488 |
| 4-Methoxy-2-sulfanylphenol | C7H8O2S | 156 |
| 3-n-Pentadecyl-2,4-dinitrophenol | C21H34N2O5 | 394 |
| (5-n-Pentadecyl-2,4-dinitro-1-hydroxy)benzene | C21H34N2O5 | 394 |
| 4à,14à,24-trimethyl-9(11)-cholesten-3á-yl acetate | C32H54O2 | 470 |
| 5-Hydroxy-3,3',4',6,7-pentamethoxy flavone-3',3',3',6,6,6-D6 | C20H14D6O8 | 388 |
| 7-Chloro-3-[3,4-dichlorophenyl]-1,10-dihydro-1,10-dihydroxy-9(2H)-acridinone | C19H12Cl3NO3 | 407 |
| β sitosterol | C32H54O2 | 470 |
Figure 10. GC chromatogram of (A) Sh-ALE and (B) Sh-SeNPs.
Download figure:
Standard image High-resolution image4. Conclusion
AgNPs and SeNPs were synthesized rapidly in a single step method using S. hispida plant ALE. The use of S. hispida plant ALE was advantageous since it is among few plants which were able to synthesis two different metal NPs efficiently. The NPs are rod-shaped with a particle size of 470 ± 20 nm for Sh-AgNPs and 120 ± 15 nm for Sh-SeNPs. Phytochemical, FTIR and GC-MS analysis revealed the involvement of flavonoids, saponin and phenolic compounds present in Sh-ALE in the formation of NPs. Also, Sh-SeNPs has been biofunctionalized by the attachment of derivatives of apigenin, quinoline and quinazoline, and synaptogenin B. Such conjugation of bioactive compounds on the surface of the Sh-SeNPs has differentiated it from Sh-AgNPs through the presence of efficient biological application as anticancer agent in vitro, anti-inflammatory activity in vitro and antibacterial agent. Further, in vivo studies are warranted to understand the biological application of Sh-SeNPs.
Acknowledgments
L Chitra acknowledges Science and Engineering Research Board, India for the award of National Post-Doctoral Fellowship (Ref. No. PDF/2015/000252). K Vennila acknowledges Periyar University, Tamil Nadu and India for the award of University Research Fellowship.