Abstract
Selenium nanoparticles (SeNPs) were synthesized through the bioreduction of sodium selenite (Na2SeO3) using gram-negative agrobacterium (AGBT) species. Subsequently, their physicochemical properties (pH, viscosity and surface tension) and medicinal activities as anti-dermatophyte against soil keratinophilic fungi at the molecular level were assessed. UV–visible and FTIR spectroscopic data of the biologically synthesized SeNPs were then recorded for confirming the presence of native biological materials adhered to nanoparticles, which are inherently required to enhance the stability and solubility through inhibition of the nanoparticle's natural aggregation and agglomeration. The λmax value between 290–300 nm in the absorption spectra of the biogenic materials in different concentrations of the Na2SeO3 corroborated the presence of SeNPs in the solution. The interaction of SeNPs in solution state was further studied through the determination of pH, viscosity and surface tension values of agrobacterium-derived SeNPs in different solvents. The pH value of SeNPs dispersed in water is reported as above 7.0 and the average viscosity, and surface tensions of the SeNPs are appeared as near to the water. The particle size distribution was further determined by DLS and the highest % of particle size of the synthesized SeNPs is found in between 200–300 nm. The anti-dermatophyte activity and molecular interaction with fungi DNA molecules were assessed providing the highest anti-dermatophyte activity at 0.1 M concentration and it is observed that the quantities and qualities of fungi DNA were affected by SeNPs. Considering all the outcomes of the studies together, our findings suggest that agrobacterium-mediated synthesis of SeNPs is dependent on bacterial metabolisms but not on the concentration of Na2SeO3 and are promising selenium-derived species with potential application in the prevention of fungal infection through denaturation of fungi DNA.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 3.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Selenium is one of the vital metalloids of considerable environmental importance in the animal and plant kingdom and naturally found in a soil, and a variety of foods, such as Brazil nuts, seafood, organ meats, to name a few. Biologically, it is an essential micronutrient of all living organisms, including prokaryotes, for the synthesis of essential amino acids, i.e. selenocysteine and selenomethionine, which are beneficial for the persistence of fertility and a healthy immune system for both women and men [1–3]. The incorporation of these selenoamino acids into protein backbone prevents cellular damage and helps in regulation of the function of the thyroid gland and proper functioning of the immune system [4]. In addition, selenium is also used as a strong anti-carcinogenic and antimicrobial agent against a different type of cancerous cells [5, 6]. Despite possessing medicinal properties in a trace amount, selenium can be toxic in excess. Thus, though various advantages of selenium element at a trace amount are ubiquitously exposed to common people of the society, the presence of high concentration of the element has been adversely affecting the health of animals and plants [7, 8]. Therefore, a group of researchers is relentlessly engaged in studies to eradicate and/or reduce the adverse effects caused by the presence of high concentration of selenium by the controlled use of its medicinally active nanoparticles [9–12]. In this regard, the development of new and sustainable synthetic routes, and characterization techniques of selenium nanoparticles (SeNPs) have become the focus of intensive research area owing to their wide range of applications in different areas, such as antioxidants [13–17], photocatalyst [18], antibacterial and anticancer activity [19, 20]. Recent literature also revealed that SeNPs are more biologically potent and less toxic in contrast to their bulk counterpart [21].
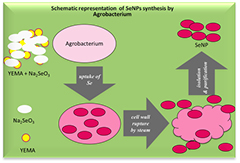
Generally, fungal infections are found in different parts of living and non-living objects, and it has become difficult to find an appropriate treatment method due to limited antifungal agents and the sudden emergence of antibiotic/antifungal resistance [22–26]. Recently, more cases are found on fungal infectious diseases, which cause significant challenges on global health at the world level, especially against the emergence of resistant dermatophyte (keratinophilic) fungi and the adverse side effects associated with prolonged use of effective antifungal treatments [15]. While SeNPs have been used for a wide range of applications including antibacterial, antioxidant and anticancer applications, but the antifungal activity study of SeNPs is limited. Thus, additional and in-depth studies are imperatively required for exploring the antifungal activity of SeNPs as a safe and potent alternative to traditional antifungal drugs. Furthermore, literature precedents have presented intensive applications utilizing SeNPs as one of the smart materials in chemistry, electronics, medicine, and biotechnology [16]. Though many physical, biological and chemical methods are published for the synthesis of the SeNPs, but biosynthetic approaches being eco-friendly and non-toxic are more focused and accepted for the practical synthesis of these nanoparticles [15]. It is relevant to mention that biomacromolecules are found to serve as a powerful biocatalyst for the synthesis of a wide range of nanoparticles. To the best of our knowledge, many biomaterials like fungus, bacteria, protozoa, plants, algae and different microorganisms are involved in the biosynthesis of nanoparticles. Among these, the bio-reduction of selenite (Se(IV)) to SeNPs is of great interest in regards to our ongoing research work as it is expeditious, concise, easy and requires no special and tedious conditions (scheme 1).
Scheme 1. SeNPs synthesis.
Download figure:
Standard image High-resolution imageInstead of working as able bioreducing agents, biomachinaries of the living organisms provide an additional support as natural stabilizers for nanoparticles [17]. The synthesis of nanoparticles using microorganisms suggested a new avenue as a possible alternative to chemical and physical approaches [19]. It is found in the literature that the microorganism assists the bio-reduction of selenite (Se(IV)) to synthesize SeNPs with the size ranging 100–500 nm [20]. It is also reported that the SeNPs have antifungal properties [19], which might be useful for diagnostic study in dermatophyte diseases as how SeNPs inhibit the growth of fungi or fungicidal effects. In this regard, the molecular aspect of SeNPs is studied in this report through interaction with DNA. It is assumed that the SeNPs will bind either with four or six molecules of DNA or RNA nucleotide at the site of phosphate ion through an ionic bond or as an antifungal agent, it might effect on cysteine and methionine amino acid of proteins by converting them into selenocysteine or selenomethionine in presence of Se-supplements. This is a pioneering attempt to evaluate the antifungal activity of SeNPs synthesized from selenite through bioreduction. In this direction, agrobacterium species are used to synthesize SeNPs by a simple biological procedure through the reduction of sodium selenite (Na2SeO3) following a simple biological protocol. In brief, at first, the bacterial cell membranes were destabilized by steam penetration followed by a high-speed centrifugation [21, 27]. This method is capable of producing higher (%) of particle size of SeNPs between 200–300 nm diameters. These SeNPs are characterized by UV–vis spectroscopy with 220–400 nm wavelengths. The average pH values of synthesized SeNPs are greater than 7.0 [28, 29]. The values of zeta potential, particle size distribution, the mobility of ionic charges and percentage of SeNPs are analyzed by DLS techniques. FTIR spectroscopic study of naturally occurring organic biomolecules adhered to SeNPs as stabilizers suggest that C–C, C–H, C–O, C–OH bonds are involved in the biological synthesis of SeNPs. The extract of SeNPs from biological sources, which reduce or inhibit the growth of keratinophilic fungi, is known as antifungal agents by denaturing or shearing DNA. These phenomena are studied by Gel electrophoresis with 1% agarose gel. In essence, this paper aimed an attempt at demonstrating the physicochemical parameters (PCP) at solution state and potential application in the prevention of fungal infection through denaturation of fungi DNA of selenium-derived nanospecies obtained via a very facile and convenient biogenic synthetic method.
2. Experimental
2.1. Material and methods
The chemicals, solvents, and reagents required for conducting the experiments were purchased from standard companies: Na2SeO3 (Hi-media), YEMA (Hi-media), SDA (Hi-media), Agar-agar (Sigma), safranin dye (Hi-media), methylene blue dye (Hi-media), lactophenol cotton blue (LPCB) dye (Hi-media), SDS(Hi-media), EDTA (Hi-media), Tris (Hi-media), β-mercaptoethanol (Sigma), phenol (Sigma), chloroform (Bengal reagent), isoamyl alcohol (Bengal reagent), ethanol (Bengal reagent), methanol (Bengal reagent), bromophenol blue dye (Hi-media), NaCl (Hi-media), ethidium bromide (EtBr) (Hi-media), agarose gel (Sigma), isopropanol (Bengal reagent), HCl (Sigma), NaOH (Hi-media), UV–vis spectrophotometer, FTIR (Parkin Elmer), survismeter (Borosil), DLS, ultrasonicator (Oscar) and digital pH meter (Chem lab).
2.2. Isolation of agrobacterium
For the isolation of agrobacterium, the soil samples were collected in sterile polythene bags from the local farming area of Betul (MP), India. To a 1.0 g of the soil sample in a test tube, 10 ml of sterile distilled water (DW) was added and shaken for 30 min. The soil sample was allowed to settle down at the bottom of the test tube for 10 min and 1 ml of suspension was inoculated into each Petri plate and then 10 ml of preheated yeast extract mannitol agar (YEMA) medium was added into each plate and the plate was kept in incubator at 28 ± 2 °C for 5–7 d till the appearance of the pink colour of few colonies on culture media [30]. After that, YEMA media was mixed with antifungal Amphotericin B and prick a pink colony from pre-culture plates and streaking on media by inoculator needle and the culture was kept at 28 ± 2 °C for 5–7 d and pure agrobacterium colonies were developed (figure 1).
Figure 1. Agrobacterium culture.
Download figure:
Standard image High-resolution image2.3. Identification of agrobacterium
A clear morphological view of bacteria by microscope provides information about cell's shape, size, color, and arrangement. An agrobacterium cell has a positive charge and called basic strain because basic dye has a negative potential [31]. For the experiment, slides were prepared and staining was performed in two ways, simple staining, and negative staining. To prepare slides, a sterilized germ-free slide was taken and one drop of DW on the center point of the slide was applied. Aseptically, a colony from culture plate by inoculator's needle near a flame under laminar air flow was taken and a loopful of culture was transferred onto a glass slide. It was mixed with one drop of DW and finally spread it in a circle to prepare a mixture with milky color. The slide was allowed to dry in the air for 10 min before heat fixation; the slide was heated gently by passing over the flame and made ready for applying dye stains.
In a simple staining, crystal violet methylene blue or 1–2 drops safranin dye were applied on dried smear and allowed to stand for 60 s. Then the entire smear was covered with staining dye. After that, the stain was gently washed off with running water, dried the slide in the air with blotting paper and examined the slide under the microscope at high resolution using the oil immersion objective lens. In negative staining process, a drop of nigrosin dye (India ink), or Congo-red was placed near the edge of a clear glass slide. Aseptically, a loopful colony was transferred to the drop of stain placed on the slide and mixed together. Both simple and negative staining of slides was examined using the oil immersion objective lens under a microscope.
2.4. Isolation of dermatophytes (keratinophilic) fungi
Different areas of Betul, MP, India, usually rich in the keratinous material were selected for the isolation of the fungi [32] from soil samples. The fungi were isolated by the hair baiting technique and spread on Sabouraud dextrose agar (SDA) media following plate method. For that, the sterile Petri dishes were half filled with approx. 10 g soil sample on each plate. A soil sample was spread over the surface of the soil and 10–15 ml of sterile water was added to the soil to facilitate germination of fungal spores with some antibiotic, such as penicillin G to prevent bacterial growth. Each Petri plate was incubated at room temperature (25 °C) in the dark place for 4–6 weeks. After that, the plates were examined routinely for the development of the mycelium. While opening the plates, we made sure that there was no free moving air, because the spores, which might be pathogenic, could be dispersed by air. Keratinous materials hair, nail, and skin were removed from plates with fungal growth or inoculum was taken and placed it on the vessel of SDA media. After 1–2 weeks, the colonies were checked and identified the fungus for preparing the pure cultures. A colony of mycelium was pricked from keratin materials and speared it on the midpoint of a plate of solidified SDA media. These materials were transferred to plate containing SDA and incubated at 28 ± 2 °C for 7 d and shown in figure 2. The microscopic studies were executed on the basis of morphological characteristics. Slide culture as a technique was applied for the identification of fungus with LPCB staining dye [33, 34]. The mycelium's prick from the culture plate by aseptic needle and placed on the midpoint of sterilized slide and spread it on the slide through the help of a needle. The mycelium was stained by one drop of LPCB dye for permanent mounting. The slide observed under the microscope at 10×, 40× and 100× magnification and equipped with oil immersion lens was shown in figure 2.
Figure 2. (a) pre mycelium grown on keratin, (b) close view of keratin with fungi, (c) overgrowth of fungi in Petri plate, and (d) microscopic view of Keratinophilic fungi at 40× magnification on SDA media.
Download figure:
Standard image High-resolution image2.5. Synthesis of SeNPs
Yeast extract mannitol broth (YEMB) (YEM without agar) in 500 ml volumetric flask was divided into 10 ml culture tubes in different concentrations of Na2SeO3 (0.1, 0.2, 0.3, 0.4, 0.5, 0.6, 0.7, 0.8, 0.9 and 1.0 M) in four replicates maintaining pH 6.8 of each culture tube using 1 N NaOH and 1 N HCl. One loopful of culture was inoculated from a pure culture of agrobacterium bacteria species and was used in experiments for the synthesis of SeNPs from Na2SeO3. Then 40 culture tubes were inoculated and the culture was placed at 28 ± 2 °C for 5–7 d in a rotary shaker incubator at 150 rpm for the growth the of agrobacterium bacteria. Agrobacterium needs an appropriate culture medium, which contains a carbon source, nitrogen source, and inorganic ions. The applied organic micronutrient source contains sufficient quantity of desired materials. After 5–7 d, each culture tube developed pink color (figure 3) with the appearance of slight turbidity indication the formation of SeNPs [9–12]. All culture tubes were removed from the incubator for tipping all the tubes approximately for 25–50 times to shake well. Then, the bacterial growth was pelleted by centrifugation at 10 000 rpm for 10 min and discarded supernatant. This process was repeated for each tube and collected the pellet from each replicate. The pellet obtained from the first 10 culture tubes were re-suspended in 10 ml DW and the pellet obtained from the second set of 10 culture tubes was re-suspended with 10 ml ethanol. The pellet obtained from the third set of 10 culture tubes was re-suspended with 10 ml methanol and the pellet obtained from the fourth set of 10 culture tubes was re-suspended with 10 ml chloroform. All four groups of 10 culture tubes containing agrobacterium cells with red SeNPs were treated with a soft heat steam to disrupt the cell wall. This process was done in an autoclave at 120 °C for 15 min to destabilize the cell wall of bacteria and released the SeNPs in aqueous and organic solvents. Subsequently, the pH values of each tube were determined. The solvent was evaporated by a water bath at 100 °C temperatures for 1–3 h to dry each group of 10 culture tubes and all tubes were kept in the freezer for characterization of SeNPs using DLS, UV–vis spectroscopy, pH meter, survismeter, FTIR, DLS.
Figure 3. (A) YEMB + agrobacterium and (B) YEMB + agrobacterium + Na2SeO3.
Download figure:
Standard image High-resolution image2.6. Characterization of SeNPs
It is ubiquitously known to us that the PCP of the bioactive materials play important roles in understanding the mechanism of interaction with physiological media, the pre-formulation study, correlating biological activities and desired therapeutic effects and in addition, it could affect the drug performance and the development of a dosage form. Thus we were interested in analyzing the PCP of the dispersed SeNPs in different solvents using pH meter, survismeter for viscosity, intermolecular forces and surface tension, UV–vis spectrophotometer, DLS, and FTIR.
2.7. pH value determination
The pH of the culture media containing Na2SeO3 and agrobacterium culture changes to alkali or acidic media because biomolecules may be acidic or basic in nature. The pH of the mixture of culture media and Na2SeO3 was maintained at 6.8 by the addition of 1 N HCl or 1 N NaOH and then agrobacterium culture was added for the synthesis of SeNPs. The pH value of each sample was analysed at a different time interval and it was observed that the pH value of each tube was increasing slowly with time.
2.8. Growth inhibition of keratinophilic fungi by using SeNPs
Agar gel well diffusion method was applied for an antifungal activity with different concentrations of SeNPs synthesized from agrobacterium. The SDA plate was prepared in aseptic condition. When the gel was solidified, then small indentations were created in the centre of the gel named as a well by using a sterile diffuser stick. Then 100 µl aliquots of the dispersed SeNPs were loaded from different concentration with the help of sterile tips of the micropipette. A colony of fungi pricked from the pre-isolated master plate and applied on media loaded SeNPs. All plates were incubated at 28 ± 2 °C for 2–3 d and after that, the plates were observed and the zone of inhibition [33–35] measured by a scale in mm range was shown in figure 4.
Figure 4. Inhibited zone by agrobacterium-derived SeNPs.
Download figure:
Standard image High-resolution image2.9. Molecular aspect of SeNPs
Molecular aspects of SeNPs [36] on keratinophilic fungi may be hypothesized as it could destabilize the cellular organelles, cell wall, protein generating factories, or it can degrade the DNA of the fungi. These assumed phenomena were supported by the following ways. Firstly, 100 µl aliquots of the dispersed SeNPs were directly applied in 100 mg amounts of fungi for 1 h before extraction of DNA. On the other hand, 100 µl aliquots of fungi-extracted DNA was treated with 10 µl of SeNPs and determined their effect on quantity, quality, and band optimization through agarose gel electrophoresis.
2.10. Extraction of DNA from fungi
The method for the mini-preparation of DNA described by Lin et al [33] is suitable for large-scale screening of transgenic plants and fungi. This method was modified and adopted as follows for the extraction of DNA from fungi. Briefly, fungi tissues were placed in a mortar and ground with 1 ml of the DNA extraction buffer and all tubes were incubated at 65 °C in a water bath for 30 min. After removing all the tubes from the water bath, they were pelleted by centrifugation at 12 000 rpm for 10 min. And the supernatant was transferred into new tubes. After that, an equal volume of phenol, chloroform and isoamyl alcohol in the ratio of 25:24:1 was added and mixed several times (approximate 50 times) gently and again pelleted them by centrifugation at 12 000 rpm for 3 min. Then upper phase was carefully transferred to new tubes. This step was repeated one more time. The DNA was precipitated by adding 500 µl of ice-cold isopropanol and placed into the tube at −20 °C for 10 min and then it was pelleted by centrifugation at 12 000 rpm for 10 min, and the supernatant was discarded. The DNA pellet was suspended twice with 1 ml of ice-cold 70% ethanol and every time it was pelleted by centrifugation at 12 000 rpm for 5 min and at the end, the ethanol was poured off. The pellet was dried in the air and dissolved in 100 µl of Tris-EDTA (TE) buffer and then 2 µl of ribonuclease A (RNase A, 8 µg ml−1) were added and the product was incubated at 37 ± 1 °C for 30 min. It was stored at −20 °C for further use. The quantity and purity of DNA were determined by spectrophotometer wherein the absorbance of spectrophotometer was set to zero at 260 nm with DNA solution only without TE buffer. The 100 µl aliquots of DNA sample were taken from pre-extracted DNA from fungi and dissolved in 5 ml TE buffer. The quantity of total extracted DNA of fungi samples was analyzed. The purity of total extracted DNA from fungi was evaluated by determination of the ratio of absorbance at 260 and 280 nm (A260/280).
2.11. Optimization of DNA band
The volume of the electrophoresis tank was measured and the mixture of 1% agarose gel w/v with 0.1% Tris/borate/EDTA (TBE) buffer v/v and 0.01% v/v of EtBr in water were cast and warmed until it became transparent. After that, the concerned volume of 1× TBE buffer in the tank was added and carefully removed the comb from the gel. Then, 12 µl of bromophenol blue dye was taken on parafilm and mixed with 8 µl of DNA with the help of micropipette. The DNA sample was taken by micropipette and loaded in well of agarose gel. The integrity of DNA was determined by horizontal agarose gel electrophoresis using the system supplied by Genei (Bangalore, India) at a constant voltage of 100 V for 30 min to 1 h. After running the dyes about half-way through the length of the gel, it was removed from the electrophoresis tank and placed on illuminator gel. Then it was switched on and the DNA band was visualized in pink color via DNA binding with EtBr, shown in figure 11.
3. Results and discussion
The SeNPs were synthesized by reduction of Na2SeO3 using agrobacterium (AGBT) in YEM media. A 100 µl culture of agrobacterium was added to each of 10 tubes containing YEM broth and a stepwise gradient of Na2SeO3 concentration ranging from 0.1 to 1.0 M. After incubation of the culture at 28 ± 2 °C for 5–7 d, a pink-colored turbid solution was observed (figure 5). All culture tubes were then centrifuged at 10 000 rpm for 10 min, and the pellet was collected and re-suspended in de-ionized water for cell disruption by using a soft heat and pleasant steam. The SeNPs were released in aqueous solvent and solvent was evaporated by a water bath to dry SeNPs (scheme 1). The quantity of synthesized SeNPs depends on the amount of Na2SeO3 metabolized by agrobacterium, not on the concentration of Na2SeO3 in solution. The formation of SeNPs is indicated by the color change of the solution. It is obvious from figure 5 that with increasing concentration of Na2SeO3, the bacterial growth was slowing down. After incubation period for 5–7 d, the synthesis of the highest amount of SeNPs was resulted from 0.1 M Na2SeO3 at 28 ± 2 °C. Thus, it is specified that with increasing amount of Na2SeO3, the synthesis of SeNPs is decreased (figure 5). The physicochemical properties, shape, size and zeta potential of the synthesized nanoparticles were determined by pH meter, survismeter [37], UV–vis spectroscopy, FTIR, and DLS.
Figure 5. SeNPs in 0.1–1.0 M Na2SeO3 solutions.
Download figure:
Standard image High-resolution image3.1. Determination of pH values of SeNPs in different solvents
It is well established and ubiquitous that the medicinal properties of the bioactive compounds depend on the pH of the medium. A report in the literature shows that the relative efficacies of the antifungal activities of the compounds could be affected by the changes of the pH of the medium containing antifungal compounds. It has been also observed from the literature that with increasing pH values, there was a progressive loss of antifungal activity of the compounds; even in some cases, the few compounds were completely ineffective in the alkaline and neutral pH [38]. With this knowledge of the above literature precedents, a sincere attempt is made to investigate the effect of different solvent on the pH of the SeNPs; the investigation data could be useful at the time of formulation and dose fixation study. It is obvious that both the surface of SeNPs and the net charge of organic molecules are extremely dependent on the pH of the solution. Consequently, the dispersive efficiency of agrobacterium-derived SeNPs is observed to depend on the nature of solvents at a suitable pH. The pH of SeNPs was studied in different solvents like DW, ethanol (E), methanol (M) and chloroform (CH). The baseline of different concentration of Na2SeO3 with YEMA media was set to pH 6.5. After 1 h, agrobacteria was added to the mixture of Na2SeO3 and YEM broth, and the pH values of the mixture were determined. It is observed that the sample marked with 0.1 M provided maximum pH 7.46 and the sample marked with 0.7 M gave minimum pH 6.73. Inoculated agrobacterium samples were incubated at 28 ± 2 °C for 5–7 d and then it was found that the pH of all samples was increasing from pH 6.5 to neutral pH 7.0. Finally, the SeNPs was synthesized, dried and dispersed in the respective solvents and pH of the individual solution was measured. It is found that the maximum pH value is 7.95 for 1.0 M (DW) and minimum pH value is 7.01 for 0.9 M (E). The average pH value was evaluated for all the solution and it was observed that the average pH value is increasing in case of Na2SeO3-YEMB < Na2SeO3-YEMB-AGBT < (M < DW < E < CH) and the respective values are 6.5 < 6.91 < 7.56 in various mediums and organic solvents. Based on this experiment, it can be concluded that at the time of SeNPs synthesis from agrobacterium, H+ ions or acidic entity will be consumed and OH− ions or basic material will be released resulting the whole medium basic in nature in any types of organic solvents (figure S1 in supplementary (stacks.iop.org/ANSN/9/015004/mmedia)).
Figure 6. Average viscosity of dispersive SeNPs in different solvents: distilled water (DW), ethanol (E), methanol (M) and chloroform (CH).
Download figure:
Standard image High-resolution image3.2. Surface tension and viscosity of SeNPs in different solvents
Surface chemistry, which is one of the important outcomes of the research findings, has a great influence in many industrial and academic fields. In chemical, material and life sciences, surface area, densities, viscosities and so on are vital physical parameters. They are steeply gaining importance in the aspect of characterization of materials at the time of their development, manufacturing, and formulation. The chemical and biological activity, dissolution, adsorption, and bioavailability of a pharmaceutical agent could be depended on the surface area, viscosity and other PCP of the material of interest. Data-based information and knowledge of surface tension, viscosity and other PCP of the interested materials are of utmost importance to overcome many challenges for manufacturing of better performing and quality of new products [39, 40]. In this direction, an endeavor has been attempted in this paper to review the application of surface tension, viscosity including other PCP of SeNPs in the interest of its pharmaceutical applications. The viscosity and surface tension are important and deciding parameters for the pharmaceutical properties of SeNPs. The determination of viscosity of SeNPs dispersed in different solvents was performed by the following formula
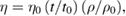
where ρ0 and ρ are the densities of solvent and solution, respectively, t0 and t are the flow times of solvent and solution, η0 and η are the viscosities of solvent and solution, respectively. Analysis of viscosity of dispersed SeNPs at various concentrations (0.1 M to 1.0 M) in DW, ethanol (E), methanol (M) and chloroform (CH) had interesting results (figure S2 in supplementary). The SeNPs dispersed in chloroform hadhighest value η = 1.3284 g cm−1 s−1 at 1.0 M and lowest value η = 1.0785 g cm−1 s−1 at 0.1 M, while the average value of viscosity (figure 6) from all samples dispersed in chloroform is η = 1.1849 g cm−1 s−1. Similarly, the SeNPs dispersed in DW had highest η = 1.053 g cm−1 s−1 at 1.0 M and lowest η = 0.8958 g cm−1 s−1 at 0.1 M, while the ethanolic SeNPs dispersion showed highest value η = 1.0977 g cm−1 s−1 at 1. M and lowest value η = 0.8777 g cm−1 s−1 at 0.1 M. In the case of a methanolic solution the highest value of viscosity was η = 1.1103 g cm−1 s−1 at 1.0 M and its lowest value was η = 0.8877 g cm−1 s−1 at 0.1 M, while the average value of viscosity from all samples of methanol was 0.9838 g cm−1 s−1. At this point, it is worthy to mention that the Mansingh survismeter provided an alternative and advantageous tool for the simultaneous analysis of PCP of the SeNPs in various solvents as an innovative experimental technique imparted in this instrument reduce the consumptions of the time, efforts, and materials used for analyzing the PCP.
The surface tension γ is another very important property of a liquid system. Similar experiments were planned for the determination of surface tension with 0.1 to 1.0 M SeNPs (figure S3 in supplementary) dispersive in DW, ethanol (E), methanol (M) and chloroform (CH) by the following formula
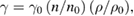
where γ0 is surface tension of solvent, n0 and n are number of drops of solvent and solution, respectively; ρ0 and ρ are density of solvent and solution, respectively. Subsequently, the assessment of average surface tension value (figure 7) in different solvents is also summarized in this report. The SeNPs dispersed in chloroform had lowest surface tension, γ = 52.13 mN m−1 and highest γ = 66.74 mN m−1 at 0.3 M and 1.0 M strength, respectively. The average values of surface tension from all samples of SeNPs dispersed in chloroform was γ = 57.57 mN m−1. The SeNPs dispersed in water provided the highest value of surface tension γ = 71.62 mN m−1 at 1.0 M and lowest value γ = 61.49 mN m−1 at 0.6 M, while the average value of surface tension from all samples was γ = 65.24 mN m−1. In case of SeNPs dispersed in ethanol the surface tension had highest value γ = 71.77 mN m−1 at 1.0 M and lowest value γ = 55.94 mN m−1 at 0.1 M, while SeNPs dispersed in methanol showed highest value γ = 65.00 mN m−1 at 1.0 M and lowest value γ = 52.07 mN m−1 at 0.3 M SeNPs.
Figure 7. Average surface tension of dispersive SeNPs in different solvents: distilled water (DW), ethanol (E), methanol (M) and chloroform (CH).
Download figure:
Standard image High-resolution image3.3. UV–Vis spectroscopic study of dispersive SeNPs in water
UV–visible spectra were recorded on a Hewlett-Packard 8451A diode array spectrophotometer. UV absorbance spectroscopy analysis of agrobacterium-synthesized SeNPs using different amounts of Na2SeO3 (0.1–1.0 M) in the wavelength range of 240 nm to 360 nm is shown in figure 8. The synthesized SeNPs were primarily characterized by UV spectrometer. The UV–vis spectra recorded at different concentration showed decreased absorption with increasing concentration of Na2SeO3 from 0.1 M to 1.0 M. The absorbance scan produced a sharp peak at ~300 nm in all different concentration indicating the formation of SeNPs.
Figure 8. UV–vis absorbance spectra of SeNPs at different concentrations 0.1–1.0 M.
Download figure:
Standard image High-resolution image3.4. FTIR spectroscopy of SeNP
FTIR measurements were carried out to obtain information about chemical functional groups present around SeNPs for their stabilization and inhibition from natural agglomeration and aggregation. In addition, this technological assistance helped understand the transformation of functional groups due to reduction process. The FTIR spectroscopy was used to characterize organic functional groups adhered to the surface of synthesized nanoparticles from biological agrobacterium. FTIR data analysis of agrobacterium-derived SeNPs dispersed in an organic solvent at a different concentration of Na2SeO3 (0.1 to 1.0 M) shows a minor change in IR frequencies. These graphs showed the typical absorption bands of C–H bonds due to the alkyl chains separated the SeNPs surface. Specifically, strong and sharp absorptions due to the asymmetrical and symmetrical stretching modes of the C–H bonds can be found in the range of 3000–2800 cm−1. Bending and rocking vibrational modes of the C–H, C–C are also accountable of the bands below 1500 cm−1. The peak for hydroxy, amine group is observed at about 3300–3600 cm−1 due to inter- and intramolecular H-bond with other –OH groups, carboxyl groups, amine and phenolic hydroxy groups. The peaks observed at about 2060 cm−1 are due to alkene and aromatic =C–H stretching frequency. The stretching frequency for alkene and aromatic C=C is observed at around 1637 cm−1. The details are shown in figure 9.
Figure 9. FTIR spectrum of SeNPs.
Download figure:
Standard image High-resolution image3.5. Determination of SeNPs size distribution by DLS
The size distribution of the SeNPs concentration in water medium was recorded by dynamic light scattering (DLS) (supplementary figure S5). Most of the nanoparticles had a diameter between 100 and 350 nm with a maximum size distribution (mean ± one standard deviation) at 19 ± 1 nm. However, a minor amount of nanoparticles were found as micrometer-sized aggregates that could not be dispersed by ultrasound. The nanoparticles were monodispersed without much aggregation indicating stabilization of the nanoparticles by naturally occurring protein, polysaccharides, and many other biomolecules as capping agents. Instead of particle size, the result also demonstrated about the size distribution, percentage of particle, viscosity, conductivity, polarity, charge, mobility and zeta potential. Herein, agrobacterium-assisted SeNPs are dispersed in water in a different concentration ranging from 0.1 to 1.0 M and the above-mentioned parameters were studied. The DLS data suggested that the size distribution of the 0.4 M concentration sample have maximum population of 25.09% at 344 nm while 0.1 M, 0.2 M, 0.3 M, 0.5 M, 0.6 M, 0.7 M, 0.8 M, 0.9 M, 1.0 M SeNPs dispersive suspension shows the size distribution with maximum percentage 18.9% of 344 nm, 11.85% of 687 nm, 23.11% of 289 nm, 17.62% of 344 nm size, 11.42% of 289 nm, 21.29% of 344 nm, 17.8% of 289 nm, 18.53% of 486 nm and 9.25% of 344 nm, respectively (table 1). The zeta potential ability of all the samples are determined and found that the highest zeta potential is −26.86 mV at 0.1 M concentration (table S1 in supplementary).
Table 1. DLS data of SeNPs.
| Concentration (M) | Size dispersity (% in nm) | Diameter (nm) | λmax (nm) |
|---|---|---|---|
| 0.1 | 18.9 | 344 | 290 |
| 0.2 | 11.85 | 687 | 290 |
| 0.3 | 23.11 | 289 | 300 |
| 0.4 | 25.09 | 344 | 290 |
| 0.5 | 17.62 | 344 | 300 |
| 0.6 | 11.42 | 289 | 290 |
| 0.7 | 21.29 | 344 | 290 |
| 0.8 | 17.8 | 289 | 300 |
| 0.9 | 18.53 | 486 | 300 |
| 1.0 | 9.25 | 344 | 300 |
3.6. Antifungal effect of SeNPs in terms of zone of inhibition
The antifungal activity of the agrobacterium-derived SeNPs from 0.1 M to 1.0 M concentration of Na2SeO3 was individually tested against the pre-isolated keratinophilic fungi. A conventional series of SeNPs were tested on SDA media used for the susceptibility test of fungi. To perform the evaluation of the biological potency of the synthesised SeNPs in terms of zone of inhibition (see figure 4), firstly, 100 µl of each agrobacterium-derived SeNPs was added with fungi in the different plate and incubated for 5 d. The diameter of the zone of inhibition in millimetre (mm) was compared to that of the control plate. Surprisingly, the maximum growth (diameter is 22 mm) of the fungi was inhibited in the sample marked with 0.1 M and the minimum (diameter is 8 mm) growth was shown in the sample marked by 1.0 M. The detail results are explained in figure 10.
Figure 10. Antifungal effect of agrobacterium-synthesized SeNPs from various amount of Na2SeO3.
Download figure:
Standard image High-resolution image3.7. Effects of SeNPs on DNA keratinophilic fungi
The method described by Weising et al [41] is reported for the study of DNA extraction and purification, including marker associated studies, fingerprinting and genome mapping. While according to the present study, the DNA extraction method from keratinophilic fungi could be achieved in less time. In this direction, the method described by Lin et al [42] was considered for completion of the extraction process as a most rapid extraction method (figure S4 in supplementary). Accordingly, DNA was extracted from the two fungi samples (sample I and sample II) and effect of SeNPs on DNA was investigated. Figure 11 illustrates the comparative studies between two replicates. The sample I and sample II are further classified into three groups A, B, and C, where A is control sample of DNA, B is SeNPs-treated DNA after extraction and C is SeNPs- treated fungi before DNA extraction. The group A is control band of both replicates with quantity 1405 µg g−1 for A-I) and 1915 µg g−1 for A-II. The quantity of the samples was 860 µg g−1 for B-I and 1310 µg g−1 for B-II (figure 12(a)) and the quality of the samples was measured and found 1.755 (A260:A280) for B-I and 1.819 for B-II. The quality of the samples was examined and found 1.836 (A260:A280) for A-I and 1.850 for A-II (figure 12(b)). Then the extracted DNA samples were treated with SeNPs resulting B-I and B-II samples. Through strong interaction with SeNPs, B-I produced contrast DNA band while B-II sample shown less interaction. Samples C-I and C-II are produced by direct treatment of SeNPs onto fungi culture followed by DNA extraction after 1 h by a similar procedure. The quantity of C-I sample was 985 µg g−1 and 2655 µg g−1 for C-II and the quality of the samples was assessed by the ratio (A260: A280) and found 2.118 for C-I and 1.916 for C-II (figures 12(a) and (b)). From the figure 11, it is clear that the DNA from sample C-I and C-II produced faint bands because DNA becomes denatured by SeNPs and thereby causing the inhibition of fungi growth.
Figure 11. Molecular aspect of SeNPs with two replicated keratinophilic fungi samples (I, II).
Download figure:
Standard image High-resolution imageFigure 12. (a) Effect of SeNPs on (a) quantity and (b) quality of fungi DNA.
Download figure:
Standard image High-resolution image4. Conclusion
In conclusion the gram-negative agrobacterium species can reduce selenite (SeO ) ions to red SeNPs, which can be added in food materials, and nutraceuticals as SeNPs can inhibit the fungal infection. This synthetic approach involves the inorganic compound Na2SeO3, which assists to produce selenium-based amino acid, selenocysteine replacing the sulphur of cysteine with selenium. In our research project, the SeNPs have been synthesized from different amounts of Na2SeO3 and its physicochemical and biological activities are thoroughly investigated. The results showed that agrobacterium-mediated synthesis of SeNPs is dependent on the rate of bacterial metabolisms not on the concentration of Na2SeO3. The synthesized SeNPs were characterized by UV–vis spectroscopy and the attached biological functional groups with nanoparticles were confirmed by FTIR spectroscopy. UV–vis spectra produced the highest peak between 280–300 nm indicating the formation of SeNPs. The particle size and morphology of the dispersed SeNPs were determined by DLS. The highest (%) of the particle size distribution of the synthesized SeNPs is in between 200–300 nm diameters. To determine the interaction of dispersed SeNPs in aqueous solution, the viscosity and surface tension were measured and found near to aqueous medium. The pH value of dispersed SeNPs in water is observed above pH 7.0. The synthesized SeNPs were further assayed against keratinophilic fungi for their anti-dermatophyte effect at the molecular level. The anti-dermatophyte aspect of SeNPs was determined through growth inhibition in a Petri dish and its binding with DNA. The highest efficacy of the agrobacterium-assisted SeNPs as anti-dermatophyte was found at 0.1 M concentration in comparison to others. DNA quantity and quality were also assessed and found that the quantity and quality of extracted DNA from fungi are affected by SeNPs. Therefore, SeNPs may be used to interact with DNA molecules at the specific site through the formation of various kinds of bonds with nitrogenous base (purine/pyrimidine) via H-bond, phosphoric acid of ribose sugar via ester bond and ionic bond to the backbone of DNA. The exploration of this mechanistic study could be one of the promising areas in future. More details are awaited at present, concerning the anti-dermatophyte effect of SeNPs on DNA at the molecular level.
) ions to red SeNPs, which can be added in food materials, and nutraceuticals as SeNPs can inhibit the fungal infection. This synthetic approach involves the inorganic compound Na2SeO3, which assists to produce selenium-based amino acid, selenocysteine replacing the sulphur of cysteine with selenium. In our research project, the SeNPs have been synthesized from different amounts of Na2SeO3 and its physicochemical and biological activities are thoroughly investigated. The results showed that agrobacterium-mediated synthesis of SeNPs is dependent on the rate of bacterial metabolisms not on the concentration of Na2SeO3. The synthesized SeNPs were characterized by UV–vis spectroscopy and the attached biological functional groups with nanoparticles were confirmed by FTIR spectroscopy. UV–vis spectra produced the highest peak between 280–300 nm indicating the formation of SeNPs. The particle size and morphology of the dispersed SeNPs were determined by DLS. The highest (%) of the particle size distribution of the synthesized SeNPs is in between 200–300 nm diameters. To determine the interaction of dispersed SeNPs in aqueous solution, the viscosity and surface tension were measured and found near to aqueous medium. The pH value of dispersed SeNPs in water is observed above pH 7.0. The synthesized SeNPs were further assayed against keratinophilic fungi for their anti-dermatophyte effect at the molecular level. The anti-dermatophyte aspect of SeNPs was determined through growth inhibition in a Petri dish and its binding with DNA. The highest efficacy of the agrobacterium-assisted SeNPs as anti-dermatophyte was found at 0.1 M concentration in comparison to others. DNA quantity and quality were also assessed and found that the quantity and quality of extracted DNA from fungi are affected by SeNPs. Therefore, SeNPs may be used to interact with DNA molecules at the specific site through the formation of various kinds of bonds with nitrogenous base (purine/pyrimidine) via H-bond, phosphoric acid of ribose sugar via ester bond and ionic bond to the backbone of DNA. The exploration of this mechanistic study could be one of the promising areas in future. More details are awaited at present, concerning the anti-dermatophyte effect of SeNPs on DNA at the molecular level.
Acknowledgments
This work was supported by the non-NET M Phil-PhD fellowship of the University Grants Commission (UGC). We wish to thank the Central University of Gujarat, Gandhinagar for providing the instrumentation and infrastructure facilities.