Abstract
Europium doped hydroxyapatite (EDA) nanorods with linoleic acid passivated silver ions on their surfaces were synthesized using facile, one-step hydrothermal route. Annealing the samples at 250 °C resulted in formation of ultra-small silver (USS) nanoparticles on the surface by nucleation through diffusion process. EDA exhibited luminescence properties due to the presence of europium ions doped on the calcium sites of hydroxyapatite. These EDA nanorods exhibited a different luminescent behavior in the presence of silver ions and USS nanoparticles. This report also demonstrates excellent biocompatibility and cytotoxicity of EDA nanorods with silver ions towards fibroblast cell lines (F929) and breast cancer cells (MCF-7). Visible and near infra-red (NIR) emissions in EDA, induced by silver ions and USS nanoparticles makes it a potential system for deep tissue imaging applications. The arrangement of USS over the EDA was tunable and hence the selectivity and enhancement of the Eu3+ ions emission can also be tuned. The multifunctional properties of this system such as its active luminescence over a wide range, its cell proliferation towards normal cells and cytotoxicity towards cancer cells shows its potential for application in cancer theranostics.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 3.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
The design of multifunctional biocompatible nano-systems is crucial in order for it to be utilized towards theranostics [1–3]. Various hybrid nano systems had been developed and analyzed in the recent past for biomedical applications. Among them nano hydroxyapatite has been widely studied owing to its biocompatible, biodegradable and accommodative nature making it a perfect host for doping various ions [4–7]. Hydroxyapatite (HAP) is an important inorganic material found in bones and teeth in the form of nano platelets giving bone its extraordinary mechanical strength in combination with other organic materials [8]. As an excellent host for lanthanides, HAP can be endowed with luminescent properties, making it a potential biocompatible system which can be exploited in the areas of sensing and tracking of particles in vivo and in vitro. Nano hydroxyapatite has also been extensively applied in hard tissue repair and replacement, drug delivery and various other biomedical applications [9–13]. It also exhibits certain unique properties such as the ability of selectively inhibiting the growth of cancer cells [14–18]. The anti-proliferation effect of nano-HAP has been observed due to the induction of apoptosis. Nanoparticle geometry also plays an important role in cell-material interaction, uptake and functioning. After absorption into the cancer cell the digested HAP nanoparticles will release calcium ions into the cytoplasm thereby disturbing the intracellular calcium hemostasis, which is crucial in regulating vital cellular functions and thus inducing apoptosis [19, 20]. The intracellular calcium concentration increases the rate of programmed cell death. The HAP nanoparticles can also activate some molecules and form free radicals, such as reactive oxygen species (ROS) resulting in the damage of the cells.
Several luminescent materials such as organic dyes, fluorescent proteins and quantum dots have been used as optical bio-probes for imaging tissues or intracellular structures and as sensors to detect biological molecules or as tracking devices [21–23], but these are prone to problems such as short life time, broad spectrum profiles, poor photochemical stability and potential toxicity to non-cancerous cells. Rare earth doped apatite nanoparticles show several advantageous properties such as strong fluorescence life time, high quantum yield, sharp emission peaks, high thermal and chemical stability, good resistance to photo bleaching and biocompatibility thus proving its effectiveness towards bio-imaging applications. It has been reported that lanthanide ions such as europium and terbium exhibited typical fluorescence for cell imaging under specific excitation wavelength, but the absorption cross section of lanthanide ions is small due to parity forbidden f–f transitions exhibiting low intense emissions and hence cannot be efficiently excited by UV light [24]. Consequently, numerous approaches such as charge transfer absorption (f–d absorption) and host absorption were used to enhance the efficiency of lanthanide ions. Surface plasmon induced enhancement and quenching of luminescence in doped apatite were investigated by coupling metal nanoparticles with lanthanide ions [25–28]. Similarly reports of enhancement due to classical energy transfer from a molecule like non-plasmonic metal nanoparticles were also made [29–31]. Among these inter particle distance and spectral characteristics play important roles in enhancing the fluorescence intensity [32].
In the present work, EDA nanorods were synthesized by a conventional hydrothermal method. This method is based on a general phase transfer and separation mechanism occurring at the interfaces of the liquid, solid and solution phases at the event of synthesis. Adding silver precursor during the process of synthesis results in the formation of silver ions on the nanorods surface. This contributes to the enhancement in the EDA emissions when excited at 395 nm. Annealing the samples at 250 °C resulted in the reduction of silver ions to ultra-small silver (USS) nanoparticles through diffusion and nucleation of silver ions present in the linoleic acid layers. The size of the thus formed nanoparticles was about 1.58 nm as observed through high resolution transmission electron microscope (HRTEM) images, which was highly stable and sparsely distributed on the surface of hydroxyapatite nanorods. The contribution of ionic silver, USS nanoparticles and linoleic acid on the emission behaviour of EDA was reported. The biocompatibility of silver ion functionalized EDA on normal fibroblast cells and cytotoxicity of these sample on breast cancer cells were studied. The results strongly suggest that these nanoparticles can inhibit cancer cell proliferation whereas it promotes the proliferation of normal cells demonstrating promising potential for cancer theranostics.
2. Experimental
2.1. Materials
All chemical reagents were used as received from standard companies without further purification. Linoleic acid (C18H32O2, 99.99%), sodium linoleate (C18H32NaO2, 99.99%), calcium nitrate hexahydrate (Ca(NO3)2·6H2O, 99.99%), sodium phosphate (Na3PO4, 99.99%), europium nitrate hexahydrate (Eu(NO3)2·6H2O, 99.99%), silver nitrate (AgNO3, 99.99%) were purchased from sigma Aldrich. Double distilled (DD) water was used throughout the experiment.
2.2. Synthesis of hydroxyapatite (HAP) and europium doped hydroxyapatite (EDA)
For the preparation of mono-dispersed hydroxyapatite, a mixture of 0.2 g of sodium linoleate, 1 ml of linoleic acid and 4 ml of ethanol were taken in a 25 ml beaker and stirred well into a homogenous mixture to which 2 ml of 0.5 M calcium nitrate was added and was stirred further for 10 min. After stirring 2 ml of 0.5 M sodium phosphate was added and stirred well. This mixture was transferred to a 50 ml autoclave sealed well and was kept at 80 °C for 17 h in a furnace. EDA was synthesized by adding the required concentration of 2 ml of europium nitrate (3 mM) before adding sodium phosphate. The particles thus prepared get settled on the bottom of the autoclave and can be collected easily by removing the supernatant. Then it was washed with ethanol and water, and then centrifuged and freeze dried to obtain a white powder.
2.3. Synthesis of silver ion functionalized EDA (EDA-S) and ultra-small silver nanoparticle functionalized EDA (EDA-USS)
EDA with surface passivated silver nanocrystals was prepared by the aforementioned procedure. Here a required concentration of 2 ml of 1 mM silver nitrate was added at the end after adding sodium phosphate to barricade the diffusion of silver ions into the Ca2+ sites of hydroxyapatite. Different concentrations of silver were added to understand the effect of silver concentration on the luminescence of EDA. The as synthesized nanoparticles with an optimized fixed concentration of europium and varying concentrations of silver were prepared. Heating silver ion functionalized europium doped hydroxyapatite (EDA-S) sample at 250 °C resulted in the formation of USS nanoparticles on the surface of EDA, here linoleic acid acts as reducing and as a stabilizing agent.
2.4. Characterization
The x-ray diffraction (XRD) patterns were recorded on a Bruker D8 advanced XRD diffractometer and the data were recorded at a 2θ value ranging from 10° to 80° using graphite monochromatized Cu-Kα radiation (λ = 0.154 05 nm). Fourier transform infrared (FTIR) spectra were recorded using Schimadzu spectrometer. All the spectra were recorded at a resolution of 4 cm−1 over a wave number range of 400–4000 cm−1 in the transmission mode. The photoluminescence emission spectra were recorded using RF-5301 PC spectrofluorometer. The morphology of the samples was characterized by using HRTEM (JEOL JEM 2100). Aqueous suspension of the prepared nanoparticles was drop casted on to a carbon-coated copper grid and the grid was air dried at ambient temperature and pressure for HRTEM analysis.
In vitro cell studies were performed as follows. The anti-proliferative activity of the synthesized nanoparticles was tested on F929 fibroblast cell line and MCF-7 breast cancer cell line using 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-tetrazolium bromide (MTT) assay. The respective cells (1 × 105/well) were placed in a 24-well plate and incubated at 37 °C with 5% CO2 condition. After the cell reaches confluence, various concentrations of the samples were added and incubated for 24 h. After incubation, the sample was removed from the well and washed with phosphate-buffered saline (pH 7.4). 100 µl/well (5 mg ml−1) of 0.5% MTT was added and incubated for 4 h. After incubation, 1 ml of dimethyl sulfoxide (DMSO) was added in all the wells. The absorbance at 570 nm was measured with UV-spectrophotometer using DMSO as the blank. Measurements were performed and the percentage of cell viability in the presence of various concentrations of the samples was calculated.
3. Results and discussion
3.1. Structural, spectroscopic and morphological analysis
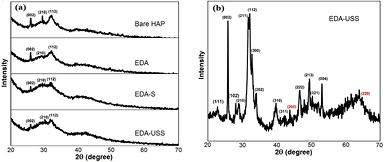
The structure and purity of the synthesized samples were investigated using XRD and FTIR. Figure 1(a) shows the x-ray diffraction profiles of bare hydroxyapatite (bare HAP), EDA, EDA-S and EDA-USS. All the diffraction peaks were indexed confirming the formation of crystalline hydroxyapatite with hexagonal structure (P63/m space group) which agrees well with the values of the standard data (JCPDS No: 09-0432). No diffraction peaks of other phases were observed indicating that the Eu2+ ions have been successfully doped in the crystal lattice of hydroxyapatite. All samples show a sharp peak at 31.76° of 2θ corresponding to that of (0 0 2) plane along the c-axis of hydroxyapatite crystals where the peaks corresponding to other planes are less intense and broader, indicating the highly unidirectional growth of hydroxyapatite nanorods. This observation was in agreement with the rod-shaped morphology observed in the HRTEM images.
Figure 1. (a) XRD patterns of bare HAP, EDA, EDA-S and EDA-USS, (b) slow scan XRD pattern of EDA-USS showing the planes of hydroxyapatite (black) and silver (red).
Download figure:
Standard image High-resolution imageIt was observed that the intensity of hydroxyapatite nanoparticles decreased with the inclusion of doped europium ions. All the peaks corresponding to that hydroxyapatite were not much evident due to the presence of linoleic acid layers on the surface of hydroxyapatite.
The presence of the linoleic acid layers was also confirmed through FTIR and HRTEM analysis. To reveal the presence of silver nanoparticles a slow scan XRD was taken for EDA-USS samples and was shown in figure 1(b). The slow scan XRD pattern of EDA-USS shows the presence of all planes corresponding to that of hydroxyapatite and two broad peaks of silver which corresponds to that of (2 0 0) and (2 2 0) planes of silver. The crystalline size of these nanoparticles was calculated as 2.9 nm from the full width half maximum (FWHM) values of these two peaks, which confirms the ultra-small size of these particles. From the XRD patterns in figure 1(a), it was observed that there were no peaks corresponding to that of silver in EDA-S, which may be attributed due to the presence of silver ions in EDA-S. The formation of USS nanoparticles on the surface of EDA, after heating was also confirmed from the HRTEM images (figure S1 (stacks.iop.org/ANSN/8/035015/mmedia)).
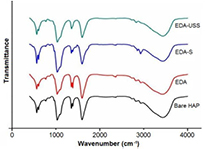
Figure 2(a) shows the FTIR spectrum of bare hydroxyapatite, EDA, EDA-S and EDA-USS. The peaks at 570 cm−1, 960 cm−1 and 1095 cm−1 correspond to the bending, symmetric and asymmetric stretching vibrations of phosphate group which confirms the formation of hydroxyapatite. The peaks at 1031 cm−1, 2736 cm−1 and 2818 cm−1 are attributed to C–O, C=O stretching groups respectively. Similarly, 1381 cm−1, 2866 cm−1, 2929 cm−1 correspond to CH3 deformation, symmetric and asymmetric stretching vibration of CH2, these peaks indicate the existence of linoleic acid covering the hydroxyapatite nanorods. P–O(H) peaks were present for all the samples indicating the bonding between hydroxyapatite and linoleic acid layers. The decrease in intensity of P–O(H) peak in the heated samples indicates the formation of USS nanoparticles within the linoleic acid surface covers thereby minimizing its interaction with the crystalline EDA. The characteristic OH bands of hydroxyapatite at 632 cm−1 are not clearly visible due to the presence of linoleic acid layers on the surface of hydroxyapatite. The peaks corresponding to C–H stretching vibrations are observed at 2734 cm−1 and 2819 cm−1. The bands observed nearly around 3000 cm−1 ascribe to the symmetric and asymmetric stretching vibrations of CH2 group. Similarly, the bands at 1029 cm−1 and 2426 cm−1 corresponds to the vibrations of carboxylic group present in linoleic acid. The absence of the peak at 1384 cm−1 for EDA-USS which corresponds to that of CH3 group confirms the oxidation of linoleic acid, which might have resulted in the reduction of the silver ions to USS nanoparticles through diffusion and subsequent nucleation while heating EDA-S samples.
Figure 2. FTIR spectra of bare hydroxyapatite, EDA and EDA-S and EDA-USS.
Download figure:
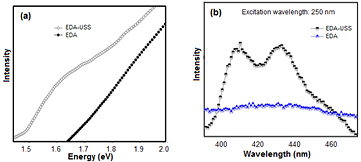
Standard image High-resolution imageThe above statement can be further confirmed by the small shift and increase in intensity of 2829 cm−1 peak for EDA-USS corresponding to that of C–H stretching vibrations. The presence of charged carboxylic groups provide stability for the USS nanoparticles formed on the surface EDA nanorods and prevents further growth of these nanoparticles to form larger aggregates. The broad peak at 3432 cm−1 is due to OH vibrations of water molecules adsorbed on the sample. The results were complemented with thermal gravimetric analysis (TGA) analysis (refer figure S1 of supporting information). Figure 3(a) shows the absorbance spectrum of EDA and EDA-USS. The broadband observed at 1.67 eV for EDA-USS arises from USS due to the formation of inter band gap between d and sp sub shells of silver, whereas this corresponding peak was absent in the case of EDA. The formation of this energy gap in USS is due to its ultra-small size. This in turn attributes molecular nature to the silver particles and makes it fluorescent. Figure 3(b) shows the emission spectra of EDA and EDA-USS at 250 nm excitation. Fluorescence emissions are observed at 407 nm and 432 nm for EDA-USS whereas they are absent in the case of EDA which confirms that these emissions are due to the presence of USS. Fluorescence of USS arises due to the recombination of sp band electrons with d band holes which is in line with the previous reports [33, 34]. Figure 4(a) shows the luminescence spectra of EDA and EDA-S. Addition of silver precursors (i.e. Presence of silver ions on the surface) shows an enhancement in the emission transitions ( ) of europium at an excitation of 395 nm. The observed enhancement in emission is due to the contribution of local field enhancement of ionic silver scattered within the linoleic acid surface [35].
) of europium at an excitation of 395 nm. The observed enhancement in emission is due to the contribution of local field enhancement of ionic silver scattered within the linoleic acid surface [35].
Figure 3. (a) UV–Vis absorbance spectrum showing the molecular behavior of USS, and (b) fluorescence of USS.
Download figure:
Standard image High-resolution imageFigure 4. (a) Emission spectra of EDA, EDA-S and EDA-USS in visible region when excited at 395 nm, (b) emission spectra of EDA in visible region when excited at 250 nm, and (c) emission spectra of EDA-USS at visible and NIR region when excited at 250 nm and 395 nm excitation.
Download figure:
Standard image High-resolution imageThe corresponding europium emissions with enhanced intensity were also observed at an excitation wavelength of 250 nm as shown in figure 4(b). Here the emissions take place through ligand mediated energy transfer mechanism. Here a radiation less electron transfer occurs from the excited triplet state of the ligand to the lanthanide ion, resulting in the excitation of 4f electrons to the excited D state, finally the lanthanide ion returns to one of the F excited ground state by radiative emission. Thus, the presence of low lying LA levels allows UV excitation leading to the emission of visible light. The energy level diagram showing the energy transitions at two different excitation wavelengths are shown in figure S2 of supporting information. Formation of USS nanoparticles on the surface of EDA-USS showed no corresponding emission associated with europium when excited at 395 nm except an emission at 790 nm as shown in figures 4(a) and (c), but all the europium transitions were observed at an excitation of 250 nm. The absence in emission corresponding to europium excitation at 395 nm after the formation of USS nanoparticles can be attributed to the involvement of energy transfer in metals [36]. However, at an excitation wavelength of 250 nm, excited emission occurs through a ligand to metal transfer process. It was observed that the intensity of these emissions is less when compared to that of EDA nanoparticles, possibly due to the involvement of linoleic acid layers in the formation of USS nanoparticles. The results were in correlation with the FTIR spectra.
One here has the opportunity to selectively tune out the wavelength of interest by engineering the arrangement of USS covers over EDA for tuning the emission behavior of Eu3+ using different excitation switch. Also, the emission of intense lines in visible and at NIR regions (known as biologically transparent window) makes the system attractive for in vitro and in vivo imaging applications, in-particular for deep tissue imaging.
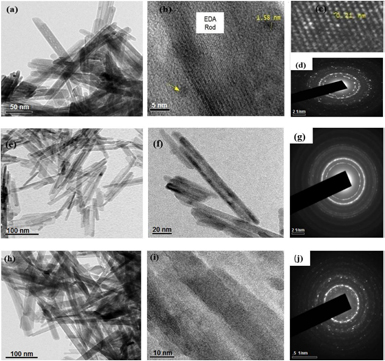
The typical HRTEM images and the corresponding selected area electron diffraction (SAED) patterns of EDA-USS, EDA and EDA-S are illustrated in figures 5(a)–(f). All the samples exhibited rod shaped morphology, the diameter of the individual nanorods were estimated to be ~5 nm with a length of about 158 nm.
Figure 5. (a) and (b) HRTEM image of EDA-USS (c) HRTEM image showing inter-planar spacing of ultra-small silver nanoparticles present in of EDA-USS (d) SAED pattern of EDA-USS (e) and (f) HRTEM image of EDA (g) SAED pattern of EDA (h) and (i) HRTEM image of EDA-S (j) SAED pattern of EDA-S.
Download figure:
Standard image High-resolution imageThe HRTEM images of EDA-USS (figure 5(b)) showed the presence of sub nanometer sized spherical nanoparticles of size ranging from 1.58 nm to 2.2 nm scattered randomly on the surface of hydroxyapatite nanorods. The lattice spacing of the sub-nanometer sized particles was found to be 0.21 nm, which agrees well with the d-spacing value of crystalline silver.
This confirms the formation of USS nanoparticles as a result of annealing. The SAED pattern of all the samples revealed the polycrystalline nature of hydroxyapatite with the EDA samples showing amorphous nature due to the presence of linoleic acid layers on the surface of apatite nanorods, whereas in EDA-USS sample the amorphous nature was not much predominant due to annealing of the samples which had resulted in the formation of USS nanoparticles. Similarly, as the result of its sub-nanometer size the particles were non-plasmonic whereas gains a molecular behavior. This can be associated from the observance of a broad absorbance peak at 775 nm, which arises due to the formation of inter band gap between d and sp sub-shells of USS nanoparticles [30].
3.2. Bio-compatible and cytotoxic investigations
The samples used for biological applications must be highly biocompatible to normal cells and hence the biocompatibility of EDA-S on fibroblast cell lines (L929) were done using MTT assay. This assay represents the metabolic activity of mitochondria present in fibroblast cells. The MTT assay is a simple non-radiative calorimetric assay to measure cell cytotoxicity, proliferation or viability. The metabolic activity and proliferation of fibroblast cells were thus measured after 24 h of culture and revealed no toxicity in the presence of various concentrations of the samples as shown in figure 6(a). Lower concentration of the sample resulted in cell proliferation indicating that the samples are highly compatible to normal cells. Similarly, cytotoxicity studies of the sample were done on breast cancer cell lines (MCF-7) using MTT assay and the cell viability in the presence of various concentrations of the samples were observed as shown in figure 7.
Figure 6. (a) Histogram showing the cell viability of (a) fibroblast cells in the presence of various concentration of sample EDA-S, and (b) breast cancer cells in the presence various concentrations of sample.
Download figure:
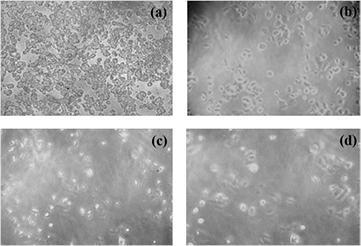
Standard image High-resolution imageFigure 7. Microscopic images of MCF-7 cell lines (a) control, (b)–(d) with increasing concentrations of EDA-S nanoparticles 1 µg ml−1, 10 µg ml−1 and 100 µg ml−1, respectively.
Download figure:
Standard image High-resolution imageThe percentage of viable cells remained more than 70% when treated with samples of concentration 15.6 µg ml−1 and with the increase in the sample concentration, cell viability percentage got decreased and only 50% of cells were viable when incubated with samples of concentration 125 µg ml−1, the percentage of cell viability further decreased with increase in concentration. A dose dependent inhibition in cell viability was observed for various concentrations with an IC50 value of 125 µg. These results indicate that EDA-S shows significant potentiality against the viability and proliferation of breast cancer cells. Similar behavior was observed for samples with USS.
Cytotoxic studies showed that the use of EDA-S induce apoptosis to breast cancer cells and were not toxic to normal fibroblast cells. The effect of EDA-S nanoparticles on the cell morphology of breast cancer cells was observed. Figure 7 shows the microscopic images of breast cancer cells incubated for 24 h in the absence (control) and presence of samples with various concentrations. A concentration dependent reduction in number of cells and significant effect on the cell morphology are observed. A clear difference was observed between EDA-S treated cells and the control. The EDA-S treated cells were denser; the morphology was changed with shorter protrusions than those in the control cells. With the increase in sample concentration a more pronounced effect on cell growth was observed indicating that these nanoparticles induce apoptosis on cancer cells. Hence these samples are highly biocompatible larger concentrations of the samples can be used for effective destruction of cancer cells.
4. Conclusion
EDA in the presence of silver ions and USS nanoparticles were prepared. Luminescence was observed at two different excitation wavelengths, due to the direct excitation of europium ions and the other due to the energy transfer between linoleic acid layer on the surface of hydroxyapatite and the europium ions. The wide range of tunable excitation makes this system an excellent probe for biomedical applications. The luminescence property of these nanoparticles can be enhanced and engineered through the size of silver clusters and nanoparticles. The silver added europium doped apatite was found to be highly biocompatible to fibroblast cells and a concentration dependent cytotoxicity was observed in breast cancer cells. These results confirm that the particles are having a strong potential for application in cancer theranostics, where diagnosis and therapy have to be a parallel process.
Acknowledgments
This work was financially supported by the Department of Science and Technology (DST), Government of India through Women Scientist Scheme A (WOS-A), Grant Number: SR/WOS-A/PS-46/2012(G). The authors would like to thank UGC-DAE center, Indore for helping in XRD measurements.