Abstract
Acid-functionalized multi-wall carbon nanotubes (MWCNTs) catalysts were prepared by a wet chemical sonication with various acid solutions, i.e. H2SO4, H3PO4, HNO3, and HCl. Sulfonic groups and carboxyl groups were detected on MWCNTs with H2SO4 treatment (s-MWCNTs), while only carboxyl groups were presented from other acid treatments. The catalytic dehydration of D-xylose into furfural was evaluated using a batch reactor at 170 °C for 3 h under N2 pressure of 15 bar. The highest furfural selectivity was achieved around 57% by s-MWCNTs catalyst, suggesting a positive role of the sulfonic functionalized groups. The effect of Co species was related to their Lewis acid property resulting in the enhancement of xylose conversion with low selectivity to furfural product.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 3.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Biomass conversion into biofuels and biochemicals has gained much interest worldwide as a result of depletion of petroleum resources [1–3]. Obtained via the dehydration of cellulosic and hemicellulosic sugars in biomass, furfural has become one of important chemical building blocks for the production of fine chemicals, potential biofuel, and fuel additives [4, 5]. In the industrial production, mineral acids i.e. H2SO4 and HCl are conventionally used as Brønsted acids for the dehydration of D-xylose into furfural as shown in figure 1 resulting in various issues such as instrument corrosion, excessive waste disposal, and high investment in catalyst recovery process [6, 7]. To overcome such problems, solid acid catalysts e.g. mesoporous silica SBA-15, titanates, zeolites, and graphene have been proposed as an alternative approach for dehydration of D-xylose [8–12]. The presence of sulfonic functional groups on the surface of SBA-15 and graphene have been reported as the Brønsted acid sites and highly active for dehydration of D-xylose [11, 13]. Moreover, these catalysts exhibit high thermal stability and negligible acid leaching effect, encouraging reusable and regeneration capabilities. Recently, the solid acid catalysts containing both Brønsted and Lewis acids have been proposed as a promising system for dehydration of sugars such as porous zirconium phosphate [14] and Sn-montmorillonite [3, 15].
Figure 1. Schematic representation of D-xylose dehydration into furfural.
Download figure:
Standard image High-resolution imageMulti-wall carbon nanotubes (MWCNTs) are one of promising carbon-based materials that can be applied for various purposes, including catalysts and catalyst supports. MWCNTs have lower cost when compared with graphene. However, commercial MWCNTs still contain undesirable contamination including amorphous carbon and residue metal catalyst particles from material processing. Thus, the purification is highly important for impurity removal using concentrated acids (H2SO4, HNO3, HCl and H3PO4) [16, 17]. Besides, this technique leads to the carboxyl group functionalization via the oxidation reaction on the surfaces of CNTs [18, 19]. In case of H2SO4 treatment on MWCNTs, the carboxyl and sulfonic groups have been proposed as a key role for acidity enhancement [20]. To the best of our knowledge, the use of MWCNTs as heterogeneous catalysts for the dehydration of xylose into furfural has been rarely presented. Acid-functionalization via oxidation reaction is one of the most commonly used methods to modify the surface of MWCNTs with carboxylic functional groups [21, 22].
Therefore this work aims to study the effect of different acid types (HNO3, HCl, H3PO4 and H2SO4) on MWCNTs functionalization and catalytic furfural production. In addition, the catalytic performance via Lewis acidity generated from Co impurity in MWCNTs was compared to that of the Brønsted acidity from acid-functionalized ones. Using commercial carbon-based materials as solid acid catalysts in aqueous system potentially provides an opportunity to utilize the sugar-based platform technology in biomass industry scale.
2. Experimental
2.1. Functionalization of MWCNTs with different acid types
The commercial MWCNTs (C-70P, Baytubes) and inorganic acid solution were obtained and used without any further purification. The acid treatment procedure on MWCNTs was performed similarly with concentrated H2SO4 (98%, Qrec), H3PO4 (85%, Univar), HNO3 (70%, Univar), and HCl (37%, Calro Erba). In short, an acid solution amount of 50 ml was mixed with 1 g of MWCNTs. The mixture was sonicated for 2 h and left for 20 h at room temperature. After that, the sample was filtered by a polytetrafluoroethylene (PTFE) membrane and then washed with deionized water until gaining rinsed water with a pH of 5. Finally, the resultants were dried in air at 100 °C for 18 h. The acid-functionalized MWCNTs by H3PO4, HCl, HNO3, and H2SO4 were named as phosphoric acid-treated MWCNTs (p-MWCNTs), hydrochloric acid-treated MWCNTs (c-MWCNTs), nitric acid-treated MWCNTs (n-MWCNTs), and sulfuric acid-treated MWCNTs (s-MWCNTs), respectively.
As Co was commonly found as an impurity in commercial pristine MWCNTs, the cobalt doping in an acid-treated MWCNTs catalyst was also prepared to compare the catalytic activity. The 3 wt% Co on s-MWCNTs catalyst was prepared by the incipient wetness impregnation method. The s-MWCNTs amount of 3 g was added to the solution of cobalt precursors, 4.576 g of Co(NO3)2·6H2O (98%, Sigma-Aldrich) in 50 ml of deionized water, and stirred at 300 rpm, 70 °C until the water was completely evaporated. After that, the sample was dried at 120 °C for 6 h and calcined at 350 °C under flowing N2 for 3 h with a heating rate of 10 °C·min−1, denoted as 3%Co/s-MWCNTs.
2.2. Catalyst characterization
Phase identity of acid-functionalized MWCNTs was analyzed by x-ray diffraction (XRD) using a Bruker D8 advance diffractometer with Cu-Kα radiation. The samples were collected at a scan step of 0.05°·min−1 from 10° to 80° and at 40 kV and 100 mA. The morphology of acid-functionalized MWCNTs was investigated by scanning electron microscope (SEM, Hitachi S-3400N) at 20 kV and transmission electron microscope (TEM, JEOL JEM-2100Plus) at 200 kV. Purity and crystallinity of the MWCNTs samples were determined by Raman spectroscopy (NTEGA spectro (NT-MDT)). Raman spectra were measured with the 632.8 nm red laser source under objective lens of 100 × magnification. Twelve scans were accumulated and the exposure time was 20 s. Functional groups on the surface of acid-functionalized MWCNTs were studied by FTIR spectroscopy (Nicolet 6700). The samples were mixed with KBr and compressed into a thin pellet before measured in a 4000–400 cm−1 wavenumber region. The Brunauer–Emmett–Teller (BET) surface area, the mean pore diameter (Dp) and the total pore volume (Vp) were estimated by N2 adsorption–desorption isotherms measured at −196 °C on a Quantachrome NOVA 2000e. Prior the measurement, the samples were degassed at 150 °C for 3 h under vacuum. The total acidic site on the surface of acid-functionalized MWCNTs was evaluated by using Boehm titration method [23]. The powder amount of 0.1 g was added to a 25 ml of 0.01 M NaOH solution. Then, the mixture was sealed and placed in a rotary shaker under 150 rpm for 24 h at 30 °C to reach an equilibrate state. Thereafter, the solution was filtrated to remove the powder sample. Filtered solution (10 ml) was titrated with a 0.01 M HCl solution to determine the left over NaOH amount. The titration was performed by the auto titration (Mettler-Toledo T50). A 0.01 M NaOH solution was also titrated as a blank test. The quantity of metal on pristine and acid-functionalized MWCNTs was analyzed using an x-ray fluorescence (XRF) spectrometer (Philips PW 2404). The Hoechst wax C micropowder was used as a binding agent to prepare MWCNTs pellets with a 10:90 weight ratio of MWCNTs to binder.
2.3. Catalytic activity testing
The catalytic performance of acid-functionalized MWCNTs was tested on the dehydration of D-xylose into furfural. The reaction was performed in a custom-made stainless steel batch reactor. D-xylose (0.9 g), catalyst (0.2 g), and deionized water (30 ml) were added into the vessel with a magnetic stirring bar. Then, the reactor was pressurized by N2 gas to 15 bars at room temperature and heated to 170 °C, following the conditions suggested in the previous report [24]. After the 3 h reaction time, the liquid product was collected and filtered with 0.22 µm nylon syringe filter. The concentration of xylose and furfural in filtrates was analyzed by a high performance liquid chromatography (HPLC, Shimadzu) equipped with a refractive index and UV–Vis detectors (210 nm) using a BIO-RAD Aminex HPX-87H column at 45 °C. A 5 mM H2SO4 solution was used as a mobile phase at a flow rate of 0.6 ml min−1. An example of the HPLC chromatogram of the product was shown in figure S1 (stacks.iop.org/ANSN/8/035006/mmedia). D-xylose conversion and furfural yield were defined [25] as the following equations:


3. Result and discussion
3.1. Characterization of acid-functionalized MWCNTs catalysts
Raman spectroscopy has been widely used to study the purity and crystallinity of carbon-based material. As shown in figure 2, the Raman spectra of all samples consisted of two major bands at 1320–1327 cm−1 and 1570–1575 cm−1 which are the representative of D-peak and G-peak, respectively. The D-peak typically indicates amorphous or disordered carbon including the defect of MWCNTs structure such as the unexpected chemical functional groups on the MWCNTs surface and metal catalyst impurities, whereas the G-peak corresponds to the graphitic carbon [26, 27]. Therefore, the ratio of the area under the D to G peaks (ID/IG) dictates the quality of MWCNTs. As summarized in table 1, pristine MWCNTs had the highest ID/IG, which presented the largest amount of amorphous carbon and impurities. For all acid-functionalized MWCNTs, smaller ID/IG suggested that the functionalization procedure by using concentrated acids probably removed the impurities and amorphous carbon.
Table 1. Physical and chemical properties of pristine and functionalized MWCNTs.
| Catalysts | ID/IG |
Cobalt concentration (wt.%) |
The total acid site concentration (µmol g−1) |
Surface area and porosity |
||
|---|---|---|---|---|---|---|
| Pore diameter (nm) | Pore volume (cm3 g−1) | SBET (m2 g−1) | ||||
| MWCNTs | 1.82 | 3.2 | 58.9 ± 42.5 | 3.3 | 0.399 | 135.3 |
| p-MWCNTs | 1.64 | 0.5 | 134.6 ± 42.5 | 3.3 | 0.465 | 132.7 |
| c-MWCNTs | 1.70 | 0.9 | 164.7 ± 50.3 | 3.2 | 0.475 | 133.0 |
| n-MWCNTs | 1.76 | 0.6 | 231.7 ± 39.0 | 3.2 | 0.461 | 130.3 |
| s-MWCNTs | 1.22 | 0.9 | 309.1 ± 34.2 | 3.3 | 0.516 | 131.0 |
aBy Raman spectroscopy. bBy XRF. cBy titration (Boehm method). dBy N2 adsorption–desorption isotherms.
Figure 2. Raman spectra of (a) MWCNTs, (b) n-MWCNTs, (c) c-MWCNTs, (d) p-MWCNTs and (e) s-MWCNTs.
Download figure:
Standard image High-resolution imageThe morphology of acid-functionalized MWCNTs was examined by SEM imaging, as shown in figure 3. The SEM image of pristine MWCNTs demonstrated some particles on the surface of the tubes, which might be metal impurities and amorphous carbon contamination from the commercial MWCNTs preparation procedure [28]. The acid functionalization of MWCNTs could help removing the impurities and encourage the process of tubes cutting and pore opening at the end of tubes [29]. The strength of the oxidation by acid-functionalization could intercalate exfoliate graphite structure of MWCNTs which were generated by the functional groups on the side wall and open end [30]. All acid-functionalized MWCNTs (figures 3(b)–(e)) showed more compact structures compared with those of pristine MWCNTs (figure 3(a)) since the carboxyl groups on the surface of MWCNTs enhance hydrogen-bonding interaction [31]. Figure 4 shows that TEM images of the carbon samples confirmed the morphology of MWCNTs before and after acid functionalization. All samples exhibited multi-walled structures with diameter in a range of 10–30 nm. As shown in figure 4(a), dark spots were observed inside the multi-walled tubes, suggesting a presence of metal impurities. Figures 4(b)–(e) show TEM images of MWCNTs treated by different acid types. It is clear that the tube structure remained almost stable as compared with pristine MWCNTs. The result suggested that the acid treatment did not remarkably destroy the tube structure of MWCNTs. The TEM images also suggested that metal impurities as observed by dark spots on MWCNTs were significantly diluted.
Figure 3. SEM images of (a) MWCNTs, (b) n-MWCNTs, (c) c-MWCNTs, (d) p-MWCNTs, and (e) s-MWCNTs.
Download figure:
Standard image High-resolution imageFigure 4. TEM images of (a) MWCNTs, (b) n-MWCNTs, (c) c-MWCNTs, (d) p-MWCNTs, and (e) s-MWCNTs.
Download figure:
Standard image High-resolution imageThe phase identity and crystallinity of acid-functionalized MWCNTs were investigated by XRD. As shown in figure 5, the diffraction peaks of all samples at around 25.8°, 42.8°, 53.8°, and 78.4° were identified as the (0 0 2), (1 0 0), (0 0 4), and (1 1 0) d-spacing planes of the hexagonal graphite, respectively. All samples with different acid treatments revealed similar XRD patterns; thus, the oxidation by concentrated acids did not affect the crystalline structure of MWCNTs. Furthermore, the XRD patterns of all samples contained a small peak at ca. 36°–37° corresponding to Co3O4 or CoO which could be the remained impurities from the production process. Hence, the purification is essential for removing the metal catalytic particles and amorphous carbon [32]. The quantity of metal residues of the pristine and acid-functionalized MWCNTs was measured by using XRF technique. As shown in table 1, cobalt species was the major metal impurity that contain in MWCNTs at 3.23 wt.%. The functionalization with all acid types could reduce the cobalt content to be lower than 1 wt.%.
Figure 5. XRD patterns of (a) MWCNTs, (b) n-MWCNTs, (c) c-MWCNTs, (d) p-MWCNTs, and (e) s-MWCNTs.
Download figure:
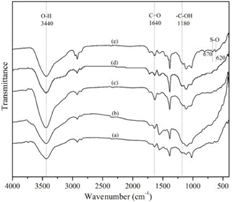
Standard image High-resolution imageThe functional groups on the surface of MWCNTs were identified by Fourier transform infrared (FTIR) spectroscopy, as presented in figure 6. All samples displayed four main peaks: ~3440 cm−1 obtained from the O–H stretching of the hydroxyl groups; ~1570 cm−1 assigned to the C=C stretching of the hexagonal structure of MWCNTs [33]; ~1640 cm−1 and ~1180 cm−1 corresponding to the C=O and –C–OH stretching of carboxyl group via acid functionalization [34]. However, the peaks of carboxylic groups also presented on the surface of the pristine MWCNTs due to manufacturer's purification [26]. For the fingerprint peaks in s-MWCNTs, sulfate groups were presented by the weak bands at ~620 cm−1 and ~670 cm−1. The results confirm that that the sulfonic acid groups are generated by concentrated H2SO4 which are in good agreement with the previous work [20].
Figure 6. FTIR spectra of (a) MWCNTs, (b) n-MWCNTs, (c) c-MWCNTs, (d) p-MWCNTs, and (e) s-MWCNTs.
Download figure:
Standard image High-resolution imageThe total acid site concentration of all samples was evaluated using NaOH titration, as shown in table 1. The acidity of all functionalized MWCNTs samples enhanced by several times from that of the pristine MWCNTs due to the carboxyl groups generated by oxidation reaction via various acid functionalization. The s-MWCNTs presented the highest acidity compared with others because of sulfonic groups besides carboxyl groups as evidenced by FTIR analysis (figure 6). Their conjugate bases (sulfonate anions) are resonance stabilized while all the resonance structures can delocalize negative charges on oxygen of sulfonic groups. Therefore, sulfonic groups are typically considered to show much stronger acidity than carboxyl groups, resulting in the significant enhancement of acidity. The specific surface area (SBET) and porous parameters of all samples were presented in table 1. The SBET and pore diameter of MWCNTs slightly decreased, while their pore volume increased after functionalization by concentrated acids. The generated acidic functional groups led to more interaction from hydrogen bonding on the surface, resulting in more compact of MWCNTs' particles which were confirmed by the SEM images (figure 3); voids between the MWCNTs' particles would contribute to the pore volume of the samples.
3.2. Evaluation of catalytic activity
The dehydration of D-xylose to produce furfural was selected to test the activity of functionalized MWCNTs as catalysts. The reactions were performed at 170 °C for 3 h under N2 pressurization. Under high temperature water system, the water as a solvent provides H+ and OH− ions which would efficiently act as acid- and based-catalysts in aqueous system [35]. Thus, high temperature water is typically an active medium for the dehydration of D-xylose into furfural. In the reaction test without catalysts, the conversion and product yield typically increased with the increase in reaction time (see figure S2). However, the product selectivity would not continuously improve since more by-products were competitively generated. Basically, side reactions, such as, isomerization, fragmentation, condensation, and resinification, could occur during the production of furfural from D-xylose resulting in the formation of small organic acids and solid precipitates (humin) as undesired by-products [36, 37]. In this work the acidity of functionalized MWCNTs catalysts was proposed to improve dehydration performance via Brønsted acid sites at the catalyst surface.
Demonstrated in table 2, D-xylose conversion catalyzed by functionalized MWCNTs was enhanced significantly in comparison to the non-catalyst system. The high furfural selectivity (~57%) was obtained on s-MWCNT which showed the highest acidity through sulfonic group as confirmed by FTIR analysis, whereas other catalysts exhibited the lower furfural selectivity by 6–14%. Compared with water system without the carbon catalysts, the xylose conversion was doubled over s-MWCNT, while the high furfural selectivity was kept constant. Sulfonic groups had more impact on furfural selectivity due to its stronger acidity character than carboxyl groups as confirmed by the FTIR and acid site concentration results. The furfural selectivity obtained from n-MWCNTs, c-MWCNTs, and p-MWCNTs catalysts was quite similar, while the conversion tendency was not simply proportional to the acidity. On the other hand, despite the lowest amount of acid sites, the pristine MWCNTs yielded higher xylose conversion, but lower furfural selectivity than acid-functionalized MWCNTs did. It was considered that the functionalized sulfonic group played a crucial role in catalyzing D-xylose dehydration to furfural. The sulfonic functional surface would presumably exhibit a similar catalysis to the high temperature water environment. Other functional groups generated by different acid treatments would catalyze the conversion of xylose with low selectivity to furfural. The result also suggested that the surface of and/or metal impurity in pristine MWCNTs would be active in converting xylose via random pathways. It was reported that catalysts with a great number of Lewis acid sites could predominantly enhance conversion of D-xylose dehydration via a xylulose intermediate [2, 38]. On the contrary, Lewis acid site would promote side reactions between xylose and furfural to form humin which is insoluble by-products. After acid-functionalization, performance of the catalysts was typically improved, while ID/IG of the catalysts decreased. Nonetheless, the correlation between ID/IG and the catalytic behaviors among the four acid-treated MWCNTs was not clear. The results were not in line with the effect of ID/IG on the catalyst performance reported by Primo et al [39] that disorder carbon structures could be active sites for the catalytic reactions and the high ID/IG ratio would give higher activity. It was considered that not only ID/IG ratio impacts the catalysis but also other catalyst properties such as acidic property, impurity elements, specific surface area and pore structures play a role on the catalyst performance. The Co impurity in pristine MWCNTs could act as Lewis acid sites, leading to the higher conversion and lower selectivity in comparison to those of the non-catalyst system. To confirm the effect of the Lewis acid sites on the xylose dehydration, a Co dopant was loaded on s-MWCNTs, namely 3%Co/s-MWCNTs. As shown in table 2, the 3%Co/s-MWCNTs catalyst could predominantly increase the xylose conversion significantly (~95%) while decreased the furfural selectivity (~30%). Therefore, the catalysts with high ratio of Brønsted acid to Lewis acid showed higher furfural selectivity and the metal species that showed Lewis acid function should be avoided from this reaction. The results are in good agreement with previous reports [38, 40].
Table 2. Catalytic activity of D-xylose dehydration at 170 °C for 3 h under N2 pressure of 15 bar.
| Catalysts | Xylose conversion (%) | Furfural selectivity (%) |
|---|---|---|
| No catalyst | 34.1 ± 6.4 | 57.6 ± 7.1 |
| MWCNTs | 80.6 ± 1.7 | 43.2 ± 2.4 |
| n-MWCNT | 73.6 ± 0.2 | 54.0 ± 2.2 |
| c-MWCNTs | 75.8 ± 3.9 | 50.2 ± 6.9 |
| p-MWCNTs | 60.9 ± 3.4 | 49.4 ± 0.2 |
| s-MWCNTs | 62.7 ± 0.4 | 57.1 ± 2.0 |
| 3%Co/s-MWCNTs | 95.0 ± 2.4 | 30.4 ± 2.7 |
4. Conclusion
The acid-functionalization successfully generated the carboxyl groups on the surface of MWCNTs. For H2SO4 treatment, the sulfonic groups were also detected in the sample via FTIR analysis, leading to the highest total acidity on s-MWCNTs. Both carboxyl and sulfonic groups could be considered as Brønsted acid sites which provide protons for catalyzing the dehydration of xylose to furfural, resulting in the enhancement of xylose conversion and furfural selectivity. The highest furfural selectivity (57%) was obtained by s-MWCNTs catalyst. Moreover, the cobalt dopant in MWCNTs, which could be considered as Lewis acid sites improved xylose conversion dramatically, but lowered the furfural selectivity.
Acknowledgments
The authors acknowledge the financial support from the National Nanotechnology Center, NSTDA, Thailand and the Thailand Research Fund (TRF—BRG6080015) to KF. This research was also supported by Collaboration Hubs for International Program (CHIRP) of Strategic International Collaborative Research Program (SICORP), Japan Science and Technology Agency (JST) and the JASTIP program-WP2 (NSTDA-Kyoto University collaboration), and the Thailand Advanced Institute of Science and Technology and Tokyo Institute of Technology (TAIST-Tokyo Tech). The authors are grateful to Dr.Teera Butburee for technical supports on microscopy imaging.
Footnotes
- *
Invited talk at 5th Thailand International Nanotechnology Conference (Nano Thailand-2016), 27–29 November 2016, Nakhon Ratchasima, Thailand.