Abstract
The magnetic nanocomposites based on bacterial cellulose (BC) matrix and ferrite (MFe2O4, M = Mn, Co, Ni and Cu) nanoparticles (NPs) were fabricated. The never-dried and freeze-dried BC nanofibrils were used as templates and a co-precipitation method was applied for NPs synthesis. The nanocomposites were either freeze-dried or annealed before subjected to characterization. The x-ray diffraction (XRD) and Fourier transform infrared (FTIR) spectroscopy showed that only MnFe2O4 and CoFe2O4 NPs could be successfully incorporated in the BC nanostructures. The results also indicated that the BC template should be freeze-dried prior to the co-precipitation process. The magnetic measurement by a vibrating sample magnetometer (VSM) showed that the strongest ferromagnetic signal was found for BC-CoFe2O4 nanocomposites. The morphological investigation by a scanning electron microscope (SEM) showed the largest volume fraction of NPs in the BC-CoFe2O4 sample which was complimentary to the magnetic property measurement. Annealing resulted in the collapse of the opened nanostructure of the BC composites.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 3.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Bacterial cellulose (BC) is a fascinating and renewable natural nanomaterial. It has received substantial interest owing to its unique structural features and favorable properties such as remarkable mechanical properties, porosity, water absorbency, moldability, biodegradability and excellent biological affinity [1–3]. Intensive research and exploration in the past few decades on BC nanomaterials mainly focused on their biosynthetic process to achieve the low-cost preparation and application in a wide range of fields such as paper making, textile industry, reinforcement materials, and medical applications [1–3]. Nevertheless, the investigations of BC in these fields have led to the emergence of more diverse potential applications exploiting the functionality of BC nanomaterials.
The hybrid nanocomposites consisting of BC nanofibers and magnetic nanoparticles (NPs) have attracted attention from many research groups due to their potential novel applications. Generally, cellulosic magnetic composites have a wide range of potential applications, for instance, electronic actuator [4, 5], magnetographic printing [4, 6], magnetic sensor [4, 6, 7], electromagnetic shielding [4, 6, 8, 9], and heavy metal adsorption [8, 10, 11]. Incorporated magnetic NP-BC has several advantages over other magnetic celluloses since BC offers much better mechanical properties. BC shows relative high Young's modulus making it both flexible and stiff membrane. Furthermore, BC-magnetic nanoparticles composites have a potential application as flexible ultra-light weight magnets [12, 13]. A previous research has reported synthesis of magnetic aerogels based on BC-ferrite nanoparticles composite [14]. Unlike other magnetic gels which are brittle in their dry states [13, 15], the BC magnetic aerogels are stiff and flexible making them very useful for practical applications.
Amongst NPs, the magnetite (Fe3O4) NP is the most widely used for incorporation in BC hybrid nanocomposites due to its generally excellent magnetic properties [4, 7–9, 11, 16]. However, in some applications other kinds of magnetic NPs may be more appropriate [6, 14, 17, 18]. Very few studies for other types of magnetic NPs in BC were reported. For examples, Olsson et al [14] reported the fabrication of magnetic aerogels by incorporating cobalt ferrite (CoFe2O4) NPs in the BC nanofibril templates. In their method, the freeze-dried BC was immersed in an aqueous FeSO4/CoCl2 solution before an immersion in NaOH/KNO3 solution. In this process, the precursors were converted into cobalt ferrite in the BC scaffold resulting in highly flexible magnetic membranes. Furthermore, Menchaca-Nal et al [18] used BC nanofibril as a template for the synthesis of cobalt ferrite nanutubes by a simple co-precipitation of FeCl3·6H2O and CoCl2·6H2O. The cobalt ferrite NPs formed into a tube shape (diameter of 200 nm) surrounding the BC nanofibers. In addition, hydrothermally synthesized barium hexaferrite nanoplates were mixed in BC solution and vacuum filtered to produce flexible magnetic papers [17]. The mechanical properties of the papers were improved by treating barium hexaferrite with silane coupling agent. On the other hand, Vitta et al [6, 19] reported the magnetically responsive BC based on crystalline metallic Ni NPs. The Ni NPs were infiltrated into the BC structure by a simple aqueous phase salt reduction method. The different drying techniques were also explored which resulted in the BC-based aerogel and xerogel with ferromagnetic behavior.
From the previous paragraph, it can be seen that very few research has been done in this field and more experimental works are still needed. Therefore, in this paper we fabricated BC-ferrites nanocomposites and investigated the phase, microstructure and magnetic properties. Four different types of cubic ferrite (MFe2O4 where M = Mn, Co, Ni, Cu) NPs were synthesized using BC (never-dried and freeze-dried states) as the template for NPs nucleation. In order to study the effect of different ferrite types on the morphology and properties, except the difference in the starting materials, other synthesis parameters were kept the same. The post-synthesis treatments (freeze-drying and annealing) of the BC-ferrite nanocomposites were also investigated.
2. Materials and methods
2.1. Chemicals
The chemical used for NPs synthesis were iron (III) chloride hexahydrate (FeCl3·6H2O, reagent grade, Sigma-Aldrich), copper (II) chloride dihydrate (CuCl2·2H2O, grade AR, QRëC), nickel (II) chloride hexahydrate (NiCl2·6H2O, grade AR, QRëC), manganese (II) chloride tetrahydrate (MnCl2·4H2O, grade AR, QRëC), cobalt (II) chloride hexahydrate (CoCl2·6H2O, reagent grade, Ajax Finechem), sodium hydroxide (NaOH, 99%, EMSURE®)
2.2. BC biosynthesis
BC was cultivated using Glucanobacter xylinum (strain TISTR 975), supplied from the Microbiological Resources Centre, Thailand Institute of Scientific and Technological Research (TISTR), in D-glucose medium. The procedure was adapted from [7]. After incubating at 30 °C under static culture for 8 d, about 1 cm thick BC hydrogels were obtained. They were boiled in de-ionized (DI) water, and soaked in 0.5 M NaOH for 15 min. The BCs were then soaked in 5 wt% NaOH for 24 h. After that, they were rinsed with DI water several times until pH of 7 was reached. Since water was not easily evaporated from the BC nanostructure, it was called the never-dried BC, which was then used as a template for synthesis of ferrite nanocomposites. Alternatively, the water molecules were replaced with alcohol, and the freeze-drying process was applied. The freeze-dried BC was also used as a template for MFe2O4 NP synthesis.
2.3. Synthesis of BC-ferrite nanocomposites
The synthesis procedure was adapted from [18]. Firstly, 0.005 mol of MCl2.nH2O (M = Mn, Co, Ni, Cu) and 0.01 mol of FeCl3·6H2O were dissolved in 100 ml of DI water. Secondly, the freeze-dried BC or never-dried BC were soaked in the prepared solution which was pre-heated to 90 °C. After 3 h, 80 ml of 1.2 M NaOH solution was added to convert the metallic ions into ferrite NPs. The process was kept for 6 h at 90 °C. Lastly, the BC-ferrite composites were rinsed with water several times to remove unwanted materials.
Two drying methods were applied to the BC-ferrite nanocomposites, namely, freeze-drying and oven-annealing. By combining the freeze-dried (F) and never-dried (N) BC templates and the 2 drying techniques (freeze-drying (F) and oven-annealing (~70 °C for 3 d) (A)), there are 4 series of samples, which were termed as FF, FA, NF and NA. The first letter represents the state of the BC template whereas the second letter represents the drying process of the BC-ferrite nanocomposites.
2.4. Characterization techniques
The phases and crystalline structures of pristine BC and BC-ferrite nanocomposites were examined using x-ray diffraction (XRD) with an x-ray diffractometer employing Cu-Kα radiation (PANalytical, Empyrean, USA). The functional groups and formation of NPs in BC nanostructure were investigated by using Fourier transform infrared (FTIR) spectroscopy (Bruker, TENSOR27, Germany). Magnetizations (M) versus magnetic fields (H) were measured using a vibrating sample magnetometer (VSM) option in the VersaLab (Quantum Design, USA) under a maximum field of 30 kOe. The morphologies of BC nanofibrils and the distribution of NPs were observed under a scanning electron microscope (SEM, FEI, Helios, USA).
3. Results and discussion
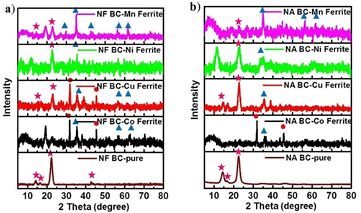
The phase and crystal structure of BC-ferrites nanocomposites can be seen from the XRD patterns (figures 1 and 2). For the pristine BC, XRD patterns showed three nicely sharp peaks indicating a good crystalline structure of BC, similar to those in literatures [4, 20, 21]. For the BC-ferrite nanocomposites, depending on the types of ferrites and the process of NP impregnation and drying, the XRD patterns varied from one sample to another. In the FF series (figure 1(a)), the strong crystalline peaks can be found for the Co ferrite and Mn ferrite samples. The Cu ferrite nanocomposite showed a peak for the ferrite phase but other unidentified peaks were still visible implying impurity phases. Almost no peak was found for BC-Ni ferrite sample, except a small hump around 35°. Similar results can be observed for the FA series (figure 1(b)), except that the peaks for NaCl salt (by-products) were observed in the Cu and Ni ferrites samples. The XRD results in figure 1 implied that our method for the synthesis of Cu and Ni ferrite NPs in the freeze-dried BC scaffold may not be suitable since the composite phases were not found simultaneously. On the other hand, Mn and Co samples showed the strong crystalline peaks for both BC and ferrite phases, making this method appropriate for fabrication the Mn and Co ferrites-BC nanocomposites.
Figure 1. XRD patterns of (a) FF and (b) FA BC-ferrites nanocomposites.  for the BC phase,
for the BC phase,  for the ferrite phase, and
for the ferrite phase, and  for the remaining salt.
for the remaining salt.
Download figure:
Standard image High-resolution imageFigure 2. XRD patterns of (a) NF and (b) NA BC-ferrites nanocomposites.  for the BC phase,
for the BC phase,  for the ferrite phase, and
for the ferrite phase, and  for the remaining salt.
for the remaining salt.
Download figure:
Standard image High-resolution imageWhen the NPs were synthesized in the never-dried BC template, the XRD patterns are shown in figure 2. A number of impurity phases can be found, particularly the NaCl peaks. The peaks for the ferrite phases were not strong, except for the NF BC-Mn ferrite sample. These results indicated that the co-precipitation of ferrite NPs were not very successful using the never-dried BC as a template. Generally, the pristine BC in the never-dried state absorbs significant amount of water [22, 23]. The water molecules are very stable unless they are exacted by force, i.e. under drying process. We hypothesize that, during the NP synthesis, it is difficult for Fe3+ and M2+ ions to infiltrate through the water barrier to reach the surface of BC nanofibers. Thus, the co-precipitation process for producing ferrite NPs is obstructed. Unlike the co-precipitation process of NPs using the freeze-dried BC template (this work and also in [14, 18]), the metallic ions can easily be adsorbed at the functional groups of BC nanofibrils. It should be noted that although it was carried out in water-based solution, water molecules were not strongly bonded with the freeze-dried BC. It is hence advised that the co-precipitation reaction should not be done using never-dried BC templates.
The presence of the strong NaCl peaks in figure 2 can be explained from the co-precipitation reaction
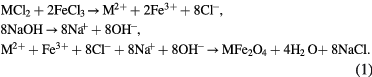
It can be seen that NaCl is the by-product of the co-precipitation reaction. However, the NaCl peaks were observed only in the samples using the never-dried BC as a template but not in the freeze-dried BC templates. We believed that for the freeze-dried BC, NaCl was mostly washed away easily in the rinsing process. On the other hand, the never-dried BC contained numerous water molecules. NaCl was dissolved in water and trapped in the BC structure which could not be easily removed by rinsing. That is the reason why the large XRD NaCl peaks were found for the never-dried BC template samples.
Another important point from XRD analysis is that though the synthesis process was the same for every sample, only the Mn and Co ferrite-BC composites showed the diffracted peaks for both ferrite and BC phases. It can be concluded that a simple co-precipitation method using chloride salts may not be suitable for preparing Ni and Cu ferrite NPs. In fact, it was reported that the as-synthesized Ni ferrite NP produced from Ni(NO3)2 and Fe(NO3)3 did not form the crystalline phase unless a high temperature annealing (>600 °C) was applied [24]. Similarly, previous research showed that CuFe2O4 phase, starting from Cu(CH3COO)2 and Fe(NO3)3, was obtained only when the NPs were heated above 600 °C [25]. It is thus assumed that the kinetics of forming the Ni and Cu ferrite phases require higher temperature than that of Mn and Co ferrites.
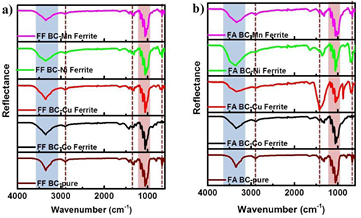
We further investigated the formation of ferrite NPs in BC structure by using FTIR spectra as shown in figures 3 and 4. Every sample in the FF series showed very similar spectra consisting of the main absorption peak at 1055 cm−1 and the broad peak at 3350 cm−1 which corresponded to the C–O–C stretching vibration and the hydroxyl group, respectively [26, 27]. Other small peaks related to C–H deformation vibration, C–H bending and stretching can be found at 810 cm−1, 1325 cm−1 and 2880 cm−1, which are the characteristic absorption peaks of BC [26, 27]. It should be noticed that the peak for –OH group was not decreased when the MFe2O4 NPs were impregnated in the BC templates. The –OH group on the BC surface was likely to attract the M2+ and Fe3+ ions in the first stage of co-precipitation process. The unreduced peak intensity of –OH group suggested that dipole-dipole interactions were the main force that anchor metallic ions onto the surface of the BC nanofibrils.
Figure 3. FTIR spectra of (a) FF and (b) FA BC-ferrites nanocomposites.
Download figure:
Standard image High-resolution imageFigure 4. FTIR spectra of (a) NF and (b) NA BC-ferrites nanocomposites.
Download figure:
Standard image High-resolution imageFor the FA series, the Mn and Co ferrite samples showed very similar FTIR spectra to the pure BC and the FF series. The abnormality was found in the Ni and Cu ferrite samples. The strong absorption peak was found at 1425 cm−1 for the Ni and Cu ferrite samples which was attributed to the unreacted NaOH precipitating agent. As mentioned earlier that the kinetics of Ni and Cu ferrite phases transformation may require high temperature process, thus, in order to obtain the Ni and Cu ferrite NPs, subsequent annealing above 500 °C should be applied [24, 25].
For the NF and NA series (figure 4), the FTIR spectra of the pristine BC resembled the FF and FA series. All BC-ferrite composite samples showed very strong absorption peaks around 1400–1500 cm−1, which corresponded to the unreacted NaOH molecules. The FTIR analysis confirmed once again that the synthesis of ferrite NPs in the presence of the never-dried BC was not achievable.
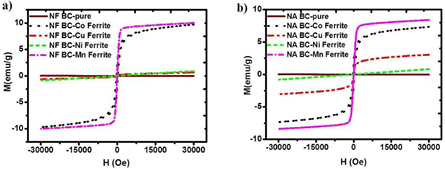
Magnetic property measurements are shown in figures 5 and 6. It is clearly seen that the magnetization values of all samples in the NF and NA series (figure 6) are much smaller than those in the FF and FA series (figure 5). The magnetic measurement supported the XRD and FTIR analysis that the ferrite NPs were not successfully formed in the never-dried BC templates, which led to low values of magnetization.
Figure 5. Magnetization (M) versus magnetic field (H) of (a) FF and (b) FA BC-ferrites nanocomposites.
Download figure:
Standard image High-resolution imageFigure 6. Magnetization (M) versus magnetic field (H) of (a) NF and (b) NA BC-ferrites nanocomposites.
Download figure:
Standard image High-resolution imageIn the case of the FF and FA series (figure 5), the saturation magnetization (Ms) was maximized for the Co-ferrite samples, followed by the Mn-ferrite sample. Besides, the Co-ferrite nanocomposites exhibited a small hysteresis loops whereas the Mn-ferrite sample showed no loop, i.e. superparamagnetism. The Ms value of the BC-CoFe2O4 nanocomposites exceeded 30 emu g−1, which was equivalent to approximately 1/3 of the Ms of the bulk cobalt ferrite [28]. It meaned the freeze-dried BC could uptake the CoFe2O4 NPs of roughly 30% of its volume. The FF BC-CuFe2O4 nanocomposites showed the superparamagnetic behavior but with relatively low Ms, whereas in the FA BC-CuFe2O4 and both FF and FA BC-NiFe2O4 samples, the ferromagnetism was almost completely diminished. These results, again, supported the XRD and FTIR analysis discussed in the previous section.
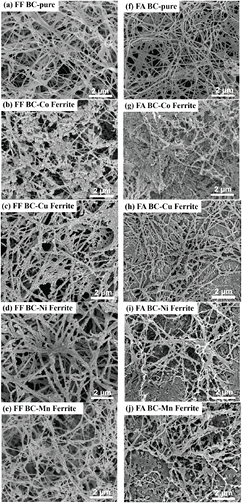
Since the magnetizations of the NF and NA series were much lower than those for the FF and FA series, the microstructural investigation was thus focused only for the FF and FA samples (figure 7). Compared between these two series, the FF samples consisted of 3D interconnected nanofibers (figures 7(a)–(e)) whereas the annealed samples (FA) resulted in some bundles of nanofibers (figures 7(f)–(j)). The FF samples also contained much higher porosity than the FA samples. The nanofiber structure of the FA BC samples appeared to be contracted and became more densely packed. The average fiber diameter of both series was less than 100 nm.
Figure 7. SEM images of the FF BC-ferrites nanocomposites ((a)–(e) in left column) and the FA BC-ferrites nanocomposites ((f)–(j) in right column).
Download figure:
Standard image High-resolution imageIn figures 7(a)–(e), the BC-CoFe2O4 nanocomposites contained significant amount of NPs compared to the other samples. The NP loaded volume, approximated by eyes, could be up to 30–40 vol%. This was corresponded to the maximized Ms for the FF BC-cobalt ferrite sample in figure 5(a). The clustering of cobalt ferrite NPs could well explain the observed hysteresis loop of BC-CoFe2O4 nanocomposite. An isolated NPs exhibit superparamagnetic behavior because thermal energy is larger than magnetic energy. A cluster of NPs has a larger volume which can sustain thermal fluctuation resulting in a hysteresis loop with finite coercivity values. Lower volume uptake of NPs was observed in the BC-MnFe2O4 sample. Well-separated NPs with small volume fraction were responsible for the superparamagnetic M–H curve with small Ms for the BC-MnFe2O4 nanocomposites in figure 5(a). For the BC-Ni and Cu ferrite nanocomposites, it was observed that the BC nanofibers were covered with the Ni and Cu pre-cursors. However, the magnetic NPs were not precipitated on the surface of BC. These images showed the strong evident for the missing XRD peaks and the non-ferromagnetic properties of the BC-NiFe2O4 and BC-CuFe2O4 samples.
The SEM images of the BC-ferrite nanocomposites in the FA series showed the more compacted nanostructures (figures 7(f)–(j)). Pore volumes were significantly reduced in all samples due to the collapse of open-spaced nanofibrils when water was evaporated. For the BC–MnFe2O4 samples, the magnetic particle size was significantly larger due to the agglomeration of NPs during the annealing process. For the FA BC-CoFe2O4 sample, it is difficult to observe individual NPs due to the collapsed BC nanostructure. However, the volume fraction of the cobalt ferrite NPs was very high, in agreement with the high value of Ms. The magnetic NPs were not clearly seen in the case of BC-Ni and Cu ferrite nanocomposites. The morphologies of the FA BC-Ni and Cu ferrite samples looked similarly to the FF samples except that the nanostructure was densely compacted. The observed microstructure supported the magnetic measurement results.
4. Conclusion
In this work we have incorporated MFe2O4 ferrite NPs in the nanostructure of BC. The XRD and FTIR results showed that the synthesized NPs using the never-dried BC as a template were not successfully fabricated. This was interpreted that the significant amount of water in the never-dried BC inhibited the co-precipitation process of magnetic NPs on the surface of BC nanofibrils. On the other hand, using the freeze-dried BC as a template, the BC-Mn and Co ferrite nanocomposites could be produced successfully. The crystalline phases of Ni and Cu ferrites were not achieved probably due to the different transformation kinetics of these ferrite species. The magnetic property measurement showed that the BC-CoFe2O4 nanocomposites exhibited the strongest magnetic signals equivalent to about 1/3 of the CoFe2O4 bulk values. The Ms of MnFe2O4 is the second highest whereas the magnetization was nearly disappeared for the other samples. Microstructural analysis using SEM evidently supported the other measurements. The largest volume fraction of NPs was found in BC-CoFe2O4 nanocomposites. The SEM also showed the collapsed nanostructure in the annealed samples due to rapid evaporation of water. It is concluded that a simple co-precipitation method using the freeze-dried BC template is the best for synthesizing BC-CoFe2O4 nanocomposites. In addition, the samples should be freeze-dried in order to preserve the opened nanostructure.
Acknowledgment
This work is supported by the Thailand Research Fund (TRF) in cooperation with Khon Kaen University (RSA5980014), the Royal Golden Jubilee Ph D Programme (PHD/0063/2558), and the Nanotechnology Center (NANOTEC), NSTDA, Ministry of Science and Technology, Thailand, through its program of Center of Excellence Network.
Footnotes
- *
Invited talk at 5th Thailand International Nanotechnology Conference (Nano Thailand-2016), 27–29 November 2016, Nakhon Ratchasima, Thailand.