Abstract
An efficient protocol for synthesis of silver nanoparticles (AgNPs) using Xanthium strumerium L. leaves was developed. This study revealed that bioactive compounds present in the extract, function as stabilizing and capping agent for AgNPs. SEM, EDX, TEM and XRD studies confirm the structure, crystalline nature and surface morphology of the AgNPs. Size of synthesized AgNPs was in the range of 20–50 nm having spherical morphology. The AgNPs were found to be toxic against pathogenic bacteria such as Escherichia coli, Pseudomonas aeruginosa and Staphylococcus aureus. The use of AgNPs as antibacterial agent is advantageous over other methods for control of pathogenic microorganisms.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 3.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Nanoscience is a boon and can bring unanticipated transformation in diverse areas of research and application [1, 2]. Nanotechnology in recent years has demonstrated its role in a number of fields such as nanomedicine, drug delivery, healthcare, food, agriculture etc [3, 4]. Metallic nanoparticles are widely used in food, cosmetics, pharmaceutical, electronics, paints, and construction materials [5]. Nanoparticles show unique electrical, light-emitting, and catalytic properties as compared to conventional bulk materials [6, 7].
Synthesis of nanoparticles with different chemical compositions, sizes, shapes, and controlled disparities is an important area of research. Several physical and chemical methods viz., chemical reduction, electrochemical reduction, photochemical reduction and heat evaporation etc have been reported for synthesis of nanoparticles [8]. Surface modifiers such as thiophenol, thiourea, marcaptoacetate etc are required in these processes, which can be harmful to biological systems as well as to environment [9–11]. Biofabrication of metallic nanoparticles by biological reducing agents including plant, bacterial and fungal extracts is an esteemed substitute to chemical and physical methods [12], although preparation of bacterial and fungal extracts requires high maintenance of cultures and are time consuming as compared to plants [13–16]. Many plant species, algae and their endophytes such as Averrhoa carambola, Solidago altissima, Chlorella vulgaris, Calophyllum apetalum and Padina tetrastromatica have been used successfully for the synthesis of silver nanoparticles (AgNPs) [17–21].
In the current investigation, a novel method for phytofabrication of AgNPs using leaf extract of Xanthium strumerium L. at room temperature (25 °C) has been described. X. strumerium (family Asteraceae) is an important plant having medicinal properties to cure malarial fever, asthma, rheumatism, leprosy, migraine, smallpox and cancer [22]. It has been well documented that medicinal properties of a plant are ascertained to the presence of specific bioactive compounds [22]. Therefore, a stable, reproducible and recyclable method for phyto-fabrication of AgNPs has been developed using the plant extract and silver nitrate solution. The fabricated AgNPs were found to be highly toxic against multidrug resistant (MDR) pathogenic bacteria.
2. Material and methods
Leaves of X. strumerium were collected from local area of Bagru town, Jaipur. Fresh leaves (5 g) were surface sterilized under running tap water for 10 min followed by soaking in liquid detergent for 2–3 min. for removal of adherent dust and other particles and washed repeatedly with sterilized deionized (DI) water. Aqueous extract of surface sterilized leaves was prepared by boiling the leaves in 100 ml deionized water for 10 min. The extract was filtered by 0.22 μm syringe filters (Millipore, India) and used immediately. Silver nitrate (AgNO3, Himedia, India) solutions of different concentrations (1–10 mM) were prepared in DI water and used for biosynthesis of AgNPs.
2.1. Biosynthesis of silver nanoparticles
Plant extract (10 ml) was mixed with 90 ml of each concentration of AgNO3 solution (1–10 mM) separately and the reaction mixtures were kept at room temperature for 4 h. for complete reduction of AgNO3 in AgNPs. After the completion of reduction reaction the dark brown mixture was centrifuged (at 12 000 rpm at 10 °C for 20 min). The pellet was thoroughly washed with sterilized DI water and suspended in known volume of DI water for characterization.
2.2. Characterization of silver nanoparticles
Primary confirmation for the synthesis of AgNPs by using X. strumerium leaf extracts was done through UV–Vis spectrophotometer (UV-1800, Shimadzu, Japan) and presence of reducing and capping agents was detected by Fourier transform infrared spectroscopy (FTIR, Thermo Nicolet, Avatar 370). The spectra were recorded between the ranges of 4000 cm−1 to 400 cm−1. Further, the surface morphology of the biosynthesized AgNPs was analyzed by scanning electron microscope (SEM-JEOL-JEM 6390, Japan). The SEM images were recorded at 40 000 × magnification operating at 20 keV. Elemental composition of synthesized AgNPs was evaluated by the energy dispersive x-ray (EDX) spectroscopy (JEOL-JED–2300). Transmission electron micrograph was also obtained using TEM (Techani G2 S Twin 200 kV) which further confirmed the morphology and size of fabricated AgNPs. Sample for TEM analysis was prepared on the carbon coated Cu grids by drop casting the nanoparticle suspension. Crystalline nature of lyophilized AgNPs was analyzed by x-ray diffraction (XRD) pattern using an XPERT-PRO diffractometer with Cu-Kα radiation.
2.3. Antimicrobial analysis
Antibacterial efficacy of biosynthesized AgNPs were carried out against different bacterial strains such as Escherichia coli (ATCC 25 922), Pseudomonas aeruginosa, (ATCC 27 853) and Staphylococcus aureus (ATCC 29 213) obtained from american type culture collection (ATCC), the Global Bio resource Center, Bangalore.
Antibacterial activity of AgNPs was analyzed by serial dilution turbidity measurement assay to determine minimum inhibitory concentration (MIC). Nutrient broth was prepared and poured 10 ml in each culture tubes followed by inoculation with 50 μl of 24 h old bacterial suspension. The stock solution of silver nanoparticles was serially diluted at 10−1, 10−2, 10−3 to 10−10 level and each tube were mixed with 0.1 ml of AgNPs of different concentrations. Optical density (OD) was recorded after every 30 min to 24 h incubation at 600 nm using spectrophotometer (Systronics, India) to measure the turbidity against a sterile blank nutrient broth. Negative control without bacterial inoculation and positive control without silver nanoparticles was also incubated.
Further, the toxicity of AgNPs was confirmed by the Kirby–Bauer disc diffusion method [23]. The bacterial suspension (24 h old) was spread out equally on to the nutrient agar (NA) plates. The sterile paper disc (whatman filter paper no. 1) of 5 mm diameter was soaked in different concentrations of AgNPs (20, 40, 60, 80 and 100 ppm) and dried for few seconds in laminar air flow cabinet. The discs with different concentrations of AgNPs were placed on to the plates and disc without any solution was used as control. The NA plates were incubated for 24 h at 37 °C. The susceptibility of the microorganism was determined by calculating zone of inhibition (ZOI).
For agar-well diffusion assay, 100 μl of 24 h old bacterial broth culture was uniformly spread over the nutrient agar plate. Then, wells were prepared in nutrient agar plates and 50 μl of different concentrations (20–100 ppm) of AgNPs were added into the wells. The ZOI were measured after 24 h of incubation at 37 °C.
3. Result and discussion
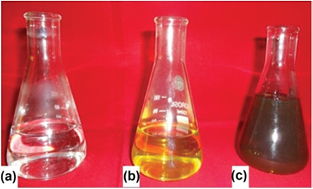
Aqueous mixture of X. strumerium leaf extract and AgNO3 solution (5 mm) showed brownish color (figure 1). The AgNPs were synthesized by the reduction of silver ions (Ag2+) in to silver metal (Ag). Change in the color is an important factor for synthesis of AgNPs as reported by several workers, which is probably owing to the surface plasmon resonance of AgNPs [24, 25].
Figure 1. Synthesis of silver nanoparticles: (a) AgNO3 solution, (b) plant extract and (c) reaction mixture.
Download figure:
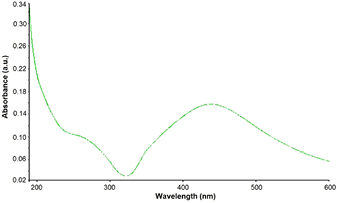
Standard image High-resolution imageThe UV–Vis spectrum showed λmax at 450 nm which is characteristic wavelength for silver (figure 2). The peak is broader indicating presence of polydispersed AgNPs in the solution owing to slow reduction rate of Ag ions in to AgNPs [26]. Earlier reports are also in the favor of the present result [27, 28].
Figure 2. UV–Vis spectrum of synthesized silver nanoparticles.
Download figure:
Standard image High-resolution imageThe nature of reducing and capping agent which is responsible for reduction and stabilization of AgNPs was predicted by FTIR spectrum (figure 3). In AgNPs solution, prominent stretches at 3170, 1593, 1388, 1261, 1072, 497 and 420 cm−1 were observed. The stretches at 3170 cm−1 are characteristic for O–H group of acids, 1593 cm−1 for C=C group of aromatic rings, 1388 cm−1 for C–F of alkyl halides, 1261 cm−1 for C–N of amines and 1072 cm−1 is due to –C–O stretch of ether linkages. These stretches confirmed the presence for bioactive compounds such as alkaloids, flavonoids and terpenoids in the plant extract which work as capping and stabilizing agents [29, 30].
Figure 3. FTIR spectrum of capped reducing compounds responsible for the synthesis of silver nanoparticles.
Download figure:
Standard image High-resolution image3.1. SEM and EDX analysis
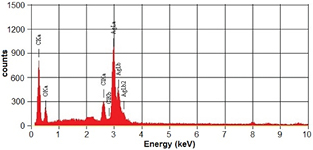
The surface morphology of AgNPs were determined by SEM. The SEM micrographs revealed spherical shape of AgNPs (figure 4). Presence of silver metal was also confirmed by EDX analysis. The EDX spectrum showed prominent signal for Ag (figure 5). According to Shankar et al [31] the capping agents cover the NPs and these NPs can remain stable for a long duration.
Figure 4. SEM image of phytofabricated silver nanoparticles.
Download figure:
Standard image High-resolution imageFigure 5. EDX spectrum of silver nanoparticles.
Download figure:
Standard image High-resolution image3.2. TEM analysis
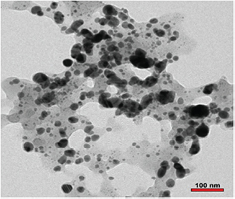
The shape and size of AgNPs was further confirmed by TEM images. TEM micrographs confirmed the size between 20–50 nm range and spherical shape (figure 6). The variation in the size is due to presence of different capping agents [32].
Figure 6. TEM image of biosynthesized silver nanoparticles.
Download figure:
Standard image High-resolution image3.3. XRD analysis
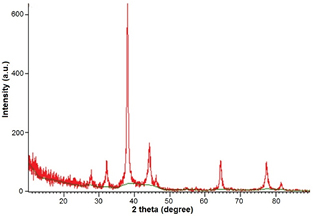
Crystalline nature of biofabricated AgNPs was further characterized by XRD. XRD pattern showed diffraction peaks of the range of 2θ (20–80°) which were corresponding to (2 6 4) (1 1 1) (2 0 0) planes (figure 7) and it revealed the FCC structure of AgNPs. FCC structure of biosynthesized AgNPs has also been reported earlier through Morganella spp. and Elaeagnus latifolia [33, 34].
Figure 7. XRD spectrum of green synthesized silver nanoparticle showing crystalline nature.
Download figure:
Standard image High-resolution image3.4. Antimicrobial activity
Antibacterial efficacy of AgNPs was evaluated to determine the toxicity against pathogenic microorganisms and the nanoparticles were found to be highly toxic at different concentrations.
In order to determine MIC, serial dilution turbidity measurement assay was carried out with a series of different concentrations (1–100 ppm) of phyto-fabricated AgNPs. The results of serial dilution method are given in table 1. The aqueous solution of AgNPs was added to the nutrient broth, and a decline in the bacterial density was recorded after half an hour. This inhibition property was measured in terms of OD. The MIC value indicated that the AgNPs inhibit the growth of test organisms at different concentrations (35–45 ppm, table 1). MIC for E. coli (ATCC 25 922) was recorded at 35 ppm while S. aureus (ATCC 29 213) and P. aeruginosa (ATCC 27 853) showed MIC at 40 and 45 ppm, respectively. Some reports have also been in favor to support present results regarding toxicity effects at different MICs of green silver nanoparticles [35, 36].
Table 1. Minimum inhibitory concentration (μg ml−1) of phyto-fabricated Ag–NPs against different pathogenic bacterial strains.
| Bacterial strains | MIC of AgNPs (μg ml−1) |
|---|---|
| E. coli | 35 |
| S. aureus | 40 |
| P. aeruginosa | 45 |
Further, zones of inhibition (ZOI) indicated that the phyto-fabricated AgNPs are efficient anti-bacterial agents against a wide range of bacterial strains (table 2). All tested bacterial strains were killed at 40 ppm concentration of AgNPs and maximum ZOI was found at 60 μg ml−1 concentration of AgNPs (table 2). Earlier reports have also confirmed that the AgNPs synthesized using biological route are effective against many pathogenic bacteria [37, 38].
Table 2. Antibacterial activity of phyto-fabricated AgNPs against different pathogenic bacterial strains by disc diffusion method.
| Bacterial strain | ZOI (mm) 20 ppm | ZOI (mm) 40 ppm | ZOI (mm) 60 ppm | ZOI (mm) 80 ppm | ZOI (mm) 100 ppm |
|---|---|---|---|---|---|
| E. coli | — | 7 ± 0.3 | 14 ± 0.4 | 15 ± 0.4 | 15 ± 0.4 |
| S. aureus | — | 6 ± 0.2 | 10 ± 0.3 | 10 ± 0.3 | 11 ± 0.4 |
| P. aeruginosa | — | 6 ± 0.2 | 9 ± 0.3 | 11 ± 0.3 | 11 ± 0.3 |
Further confirmation of antimicrobial efficacy of AgNPs was performed using agar-well diffusion assay. The results of this assay showed similar result to disc diffusion method (table 3).
Table 3. Antibacterial activity of phyto-fabricated Ag–NPs against different pathogenic bacterial strains by agar-well diffusion assay.
| Bacterial strain | AgNPs 50 μl at different concentrations (ppm) | ||||
|---|---|---|---|---|---|
| ZOI (mm) 20 ppm | ZOI (mm) 40 ppm | ZOI (mm) 60 ppm | ZOI (mm) 80 ppm | ZOI (mm) 100 ppm | |
| E. coli | — | 9 ± 0.3 | 17 ± 0.4 | 17 ± 0.4 | 18 ± 0.4 |
| S. aureus | — | 9 ± 0.2 | 12 ± 0.3 | 13 ± 0.3 | 15 ± 0.4 |
| P. aeruginosa | — | 8 ± 0.2 | 10 ± 0.3 | 13 ± 0.3 | 14 ± 0.3 |
The exact mechanism behind antimicrobial activity of metallic nanoparticles is not clearly known and is a contested matter. However, there are many concepts regarding the action of nanoparticles to inhibit the growth of microorganisms [14, 16]. It has been reported recently that silver nanoparticles have the ability to bind with the bacterial cell wall and consequently penetrate it, thereby causing structural changes in the cell membrane such as the permeability of the cell membrane and cell death. According to Danilcauk et al [39] nanoparticles accumulate on the cell surface, and enter by endocytosis. Another mechanism of cell death is the formation of free radicals by AgNPs. These free radicals have the capability to damage the cell membrane and make it porous, which can ultimately lead to cell death [40].
4. Conclusion
Synthesis of AgNPs using plant extract is a revolutionary approach in the field of nanobiotechnology because it is efficient, reproducible and ecofriendly. In the current investigation leaf extract of X. strumerium was used to produce stable AgNPs followed by characterization by different techniques such as UV–Vis spectroscopy, FTIR, SEM, EDX and XRD. The nanoparticles were found to be very effective against pathogenic bacteria and can be used as an alternative of antibiotics although the toxicity of nanoparticleas on the healthy cells has not been studied in detail yet and there is an urgent need to investigate the toxicity of NPs against the normal and healthy cells of organisms.
Acknowledgment
Authors are grateful to Prof. Sandeep Sancheti for providing basic facilities for this research work.