Abstract
Transition metal oxides with a general formula AxMaOb (A = Li, Na, M = transition metal) constitute a group of potential electrode materials for a new generation of alkaline batteries. This application is related to the fact that these compounds can reversibly intercalate high amounts of alkaline ions (1 or more moles per mole of MaOb) already at room temperature, without significant changes in their crystallographic structure. The author of this work basing on her own investigations of AxMaOb (A = Li, Na; M = 3d, 4d, 5d) has demonstrated that the electronic structure of these materials plays an important role in the intercalation process. Electronic model of intercalation process is presented. Author's studies show that electronic structure 'engineering' is an excellent method of controlling properties of the cathode materials for Li-ion and Na-ion batteries, changing their unfavorable character of the discharge curve, from step-like to monotonic, through modification and control density of states function of a cathode material.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 3.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Lithium batteries are currently the fastest growing technology of energy storage nowadays, intended mostly for mobile devices but also for transportation, energy back-up for production lines, energy storage in renewable energy systems and smart grids [1]. The important issue related to application of Li-ion batteries in cars is related to the safety problem. Much higher energy density of the batteries for electric or hybrid cars, as compared to the case of portable electronics, forces very strict safety standards, which have to be fulfilled. This leads to a constant search for more stable cathode materials, which demonstrate superior structural, thermal stability and compatibility with organic electrolytes.
Operation of Li-ion batteries is based on lithium intercalation into transition metals compounds MaXb, (M–transition metal, X = O, S) which involves both ions and electrons [1]
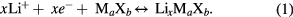
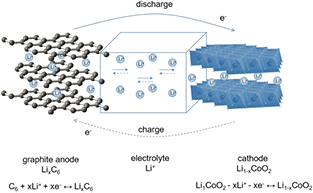
In this reaction, the energy of deep energy levels of the d-type electrons in transition metal compounds, which have a value of several eV/atom, offers the possibility of energy storage in the order of several hundreds of W h kg−1, which enables manufacturing of energy storage devices with high volumetric and gravimetric energy density. The mixed ionic-electronic conductivity is indispensable for this process. Figure 1 schematically illustrates the working mechanism of commercial LixC/Li+/Li1−xCoO2 lithium battery. While charging the cell, lithium ions are deintercalated from the LiCoO2 cathode material through the electrolyte and are introduced into the anode material—graphite. At the same time equivalent number of electrons is transferred from the cathode to the anode via external circuit. In the process of discharge, the reverse process occurs. Lithium ions are transported from graphite through the electrolyte into the Li1−xCoO2 cathode material. Electrons in the external circuit perform useful work.
Figure 1. Operation of the commercial LixC6/Li+/Li1−xCoO2 battery.
Download figure:
Standard image High-resolution imageExtended studies point that it is the cathode which limits principal parameters of lithium batteries, such as density of energy and power, while graphite anode restricts mainly the charging rate of the cell due to destruction of the anode-electrolyte interphase (solid-electrolyte interphase, SEI) [1]. Current density of the cell is determined by ionic-electronic transport properties of cathode material, whereas number of charge/discharge cycles significantly depends on the processes occurring at the interfaces of electrode material/electrolyte. Safety in use of the cell depends on the thermal and chemical stability of the electrode materials and electrolyte.
Currently, in commercial technology of Li-ion batteries, in addition to LiCoO2, also LiCo1−yNiyO2 and LiMn2O4-based materials are used as oxide cathodes. However, they exhibit significant limitations: low cyclic reversibility associated with inadequate chemical stability, high cost and toxicity. Specific capacity of LixCoO2 is equal to 140 mA h g−1, which corresponds to half of the theoretical value. For the lithium content x < 0.5 the structural and electrochemical stability of the material deteriorates. Moreover, cobalt is expensive and toxic element. Another isostructural cathode material–LiNiO2 oxide, possesses a high capacity of 200 mA h g−1, nevertheless it has also significant disadvantages. One of its limitations is the difficulty of obtaining a ordered material with layered structure, for this system cation mixing is observed (represented by the formula Li1−zNi1+zO2) [2]. This phenomenon is detrimental to the transport and electrochemical properties, as Ni2+ in the Li+ positions blocks fast lithium diffusion paths, reducing the capacity. Another drawback of this oxide is structural instability associated with phase transitions occurring during the process of charge/discharge [3], and similarly to LiCoO2, loss of oxygen from the structure for high degree of lithium deintercalation and its reaction with the electrolyte [4].
2. New tool in the development of functional materials for Li-ion and Na-ion batteries
Long time experience of author on the electrode materials for lithium batteries (over 100 publications with IF) have shown a close correlation between the electronic and the electrochemical properties of the cathode material. Figure 2 shows the concept of research on electrode materials for lithium batteries.
Figure 2. The concept of research on electrode materials for lithium batteries.
Download figure:
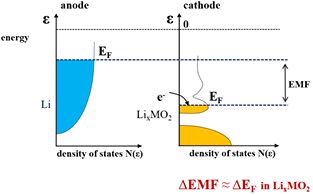
Standard image High-resolution imageWorks by Molenda on various electrode materials: LixTiS2 [5], LixCoO2 [6], NaxCoO2−y [7], LixNiO2 [8], LixVO2 [9], LixWO3 [10], Lix(Mn, Fe, Co, Ni, Cu)2O4 [11], Li(Fe, Co, Ni, Mn, Zn)PO4 [12] lead to the development of electronic model of the electrochemical intercalation process that allows to predict and design properties of materials for lithium batteries. Figure 3 shows the electron (energy versus density of states) diagram of the Li/Li+/LixMO2 cell illustrating the difference in chemical potential of electrons in the cathode and anode materials, and the resulting electromotive force of the cell.
Figure 3. The density of states of LixMO2 and lithium illustrating the difference in chemical potential of electrons and resulting electromotive force of Li/Li+/LixMO2 cell.
Download figure:
Standard image High-resolution imageThe change of the electromotive force of the Li/Li+/LixMO2 cell, wherein the potential of the anode is fixed (constant concentration of Li+ ions in the electrolyte) corresponds to the variation of chemical potential of electrons (variations of the position of the Fermi level) in the cathode material, because the change in chemical potential of lithium ions in intercalated structure is small, of the order kBT (kBT at room temperature is ~0.025 eV), which is significantly smaller than the change in chemical potential of electrons which may be of the order of bandwidth, i.e. 1 eV or more [7].
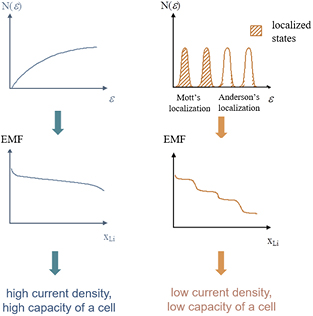
The shape of density of states function (DOS) determines the shape of discharge curve (figure 4). Monotonic density of states at the Fermi level of the cathode material and delocalized states, leads to a monotonic discharge curve (figure 4(a)), favorable from the application point of view. On the other hand, a peaked density of states leads to the step-like character of the discharge curve (figure 4(b)). Occurrence of localized electronic states near Fermi level in this case constitute a kinetic barrier for lithium intercalation/deintercalation (ambipolar diffusion of lithium ions and electrons), which effectively lowers current density and does not permit to use theoretical capacity of the cathode material. The nature of the electronic states near Fermi level of the cathode material is crucial for the effectiveness of the intercalation process, because charge transfer is performed at the Fermi level.
Figure 4. The expected character of the discharge curve (OCV) of the Li/Li+/LixMO2 cell, depending on the electronic structure of the cathode material (monotonic (a) and step-like (b)).
Download figure:
Standard image High-resolution image3. Enhancement of commercial Li1−xCoO2 cathode material and NaxCoO2−y
3.1. Li1−xCoO2
Studies by author of this paper [6] on the layered lithium cobalt dioxide showed that the observed insufficient structural and electrochemical stability of this important material is related to the overlapping of the top of 2p oxygen band and 3d cobalt band. For lithium content xLi < 0.5 during deintercalation process the electrons are extracted from 2p oxygen band (O2− ions oxidize and leave the structure). These unfavorable electronic structure properties are responsible for the serious drawback for the performance of the LiCoO2 based cathode material resulting from the structural instability related to loosing of oxygen, and its exothermic reaction with organic electrolyte leading to battery explosion. This is the reason that commercial LiCoO2 cathode working in the limited lithium range LiCoO2 ↔ Li0.5CoO2 used only 50% of its theoretical capacity. Author research aiming to understating, control and improvement of the structural and electrochemical stability of the lithium cobalt dioxide based cathode material through the modification of its electronic structure (shift the position of the Fermi level outside the 2p oxygen band) by substitution in the cobalt sublattice with Ni, Mn, Cu, Ti, Al and other elements, seem to be breakthrough in the development of functional and safety properties of the layered oxide cathode materials for Li-ion batteries. Figure 5 presents correlation between electronic structure, transport properties and discharge curve of Li1−xCoO2 and the improvement of its electronic structure by doping with Ni and Mn.
Figure 5. Correlation between electronic structure and electrochemical properties of Li1−xCoO2. (a) Charge curve (OCV) for Li/Li+/Li1−xCoO2 cell, (b) temperature dependence of electrical conductivity of Li1−xCoO2, (c) and (d) electronic structure of Li1−xCoO2 and its modification by Ni and Mn doping (e).
Download figure:
Standard image High-resolution image3.2. NaxCoO2−y
Recently NaxCoO2−y attracts enormous attention due to its unique electronic and magnetic properties. It exhibits superconductivity below 4 K, it is promising thermoelectric material and present unfavorable step-like character of the discharge curve of Na/Na+/NaxCoO2−y cell, its nature is controversial problem in the world literature. Author studies [7] of the structural, transport and electronic specific heat properties of NaxCoO2−y cathode material in the characteristic points of the discharge curve i.e. on the pseudo-plateaus and on the potential jumps, show non-monotonous variations of its transport properties (metallic/semiconducting/metallic/semiconducting/metallic) during sodium intercalation, what indicate that the density of states at the Fermi level is spiky (figure 6(a)).
Figure 6. Correlation between electronic structure and electrochemical properties of NaxCoO2−y cathode material. (a) Electronic model of Na/Na+/NaxCoO2−y cell and electronic structure of NaxCoO2−y; (b) step-like character of the discharge curve (OCV) reflecting step-like variations of the Fermi level in NaxCoO2−y during intercalation process, and (c) temperature dependence of electrical conductivity of NaxCoO2−y after partial intercalation of sodium [7].
Download figure:
Standard image High-resolution imageIt was evidenced that the origin of the observed step-like character of the discharge curve of NaxCoO2−y is due to the step-like character of the Fermi level variations in the specific features of its electronic structure, arisen from the presence of the oxygen vacancies and sodium ordering [7]. In this work we undertaken attempt to improve electrochemical properties of NaxCoO2−y by partial substitution of Co with Mn in order to achieved continuous density of states, monotonic variation of the Femi level and consequently advantageous monotonic character of charge/discharge curve.
4. Conclusion
Electronic structure 'engineering' is an excellent method of controlling properties of cathode material for Li-ion and Na-ion batteries changing their unfavorable character of the discharge curve, from step-like to monotonic, through modification and control DOS of a cathode material. Control over the processes occurring in the Li-ion and Na-ion batteries requires an interdisciplinary approach and the use of sophisticated techniques. Cooperation in the field of physics, chemistry and electrochemistry of solids, broadly defined materials science and modelling of materials, including electronic structure calculations, modelling of structural and chemical stability of electrode materials is indispensable.
Acknowledgment
The project was funded by the National Science Centre Poland (NCN) on the basis of the decision number UMO-2015/19/B/ST8/00856 and Polish Ministry of Science and Higher Education, under project AGH No. 11.11.210.911.