Abstract
This study aims to enhance the mechanical properties, thermal stability, weathering resistance and antibacterial property of a styrene acrylic polyurethane coating by adding rutile titania dioxide (R-TiO2) nanoparticles in coating formulation. The styrene acrylic polyurethane/R-TiO2 nanocomposite had been prepared by using ultrasonication. The effects of nanoparticles on the mechanical properties, thermal stability and weathering resistance of as-prepared coatings were investigated by using the adhesion strength and ball impact tests, the Fourier transform infrared and UV–vis analyses, thermogravimetric analysis (TGA), and UV/condensation weathering chamber equipped with UVA-340 fluorescent lamps, respectively. The disperse quality of nanoparticles in the coating was examined by using the field emission scanning electron microscope (FESEM). The mechanical test results showed that suitable content of R-TiO2 nanoparticles in the nanocomposite coating was 2 wt%. The FESEM images indicated that the nanoparticles were dispersed homogeneously into the entire volume of the coating. For the nanocomposite prepared by 3 h of ultrasonication, the average size of nanoparticles was in range of 40–50 nm. The ball impact and adhesion tests showed that the incorporation of nanoparticles into the coating significantly enhanced the impact strength from 120 to 145 kg cm and increased the adhesion from level 1 to level 0. The TGA test illustrated that in presence of nanoparticles, the decomposition temperature of coating increased from 146.9 °C to 154.21 °C. For the temperature at 50% loss in mass (T50%), it was found that the T50% of the neat coating is 351.86 °C. Adding the 2 wt% R-TiO2 nanoparticles into coating increased the T50% value to 360.06 °C. After UV/condensation accelerated weathering test (30 cycles), the significant improvement in weight loss, impact strength and adhesion of the neat coating was observed with the presence of nanoparticles. The antibacterial test showed that in the nanocomposite coating, R-TiO2 nanoparticles exhibited their photocatalytic effect in the inhibition against E. coli bacterial growth. Incorporating 2 wt% of R-TiO2 nanoparticles into the coating reduced the bacterial concentration by 6.1% after 60 min of culture.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 3.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Acrylic polyurethane coatings are recommended in many applications due to their excellent chemical and weathering resistance compared to other resin systems. These applications include automotive clearcoats, industrial maintenance topcoats, general metal, wood furniture and flooring. The most common coating type is two-component, where an acrylic polyol solution is mixed with a polyisocyanate solution just before used and applied to the substrate. The coating then cures by a combination of solvent evaporation and chemical crosslinking to form a durable urethane bond. These two-component polyurethane systems are the dominant technology in automotive refinishing since they can be cured at low temperatures and the coatings have good physical properties. A wide range of acrylic polyurethane coatings can be produced using various kinds of polyisocyanates and polyols. However, for exterior application exposure, acrylic polyurethane coatings have poor weather durability, due to the urethane crosslinks in the polymer matrix are susceptible to the photooxidation and hydrolysis [1–4]. In recent years, researchers have tried to resolve these disadvantages through different methods. Among that, addition of pigments, UV stabilizers (organic UV absorber, hindered amine light stabilizer (HALS)) or nanoparticles (nano-clays, nano-SiO2, carbon nanotubes, nano-TiO2, nano-ZnO...) into the formulations of coating is one of the most effective ways to enhance not only the mechanical and anti-corrosion properties, but also the thermal resistance and photostability of coating [5–13].
Nowadays, nanotechnology is regarded as one of the key technologies of the future. The rapid development of nanotechnology allowed implementation of metal oxide nanoparticles in coatings and to enhance properties of coating without significant influence on its transparency [14, 15]. Among that, TiO2 nanoparticles have attracted great attention and development in coating applications, due to their dual reactions against the light radiation: (1) as the photoabsorber, and (2) as the photocatalysts. As a photoabsorber, TiO2 nanoparticles exhibited the superior chemical stability under UV radiation and at high temperature compared to other organic UV absorbers. For outdoor exposure, since TiO2 nanoparticles did not decompose and did not migrate in coating during outdoor weathering, it might offer the long lasting UV protection for coating. It was recognized that TiO2 nanoparticles with a higher specific surface area not only serve as better nanofillers for the polymer matrix, but also more actively participate in the protection against UV radiations, compared to traditional TiO2 microparticles [14–24]. On the other hand, as a photo-catalysts, the incorporation of TiO2 nanoparticles into the organic coatings could exhibit the antibacterial [25] or self-cleaning [26] properties. It was reported that TiO2 nanoparticles had a broad spectrum of activity against microorganisms, including gram-negative and positive-bacteria and fungi. In addition, the organic molecules of contaminants on coating surface could be destroyed by free radicals, forming under UV radiation. Moreover, under UV radiation, the surface of nanocomposite coatings can become more hydrophilic behavior, due to of the formation of photooxydation products on sample surface. Thus, during the process of spreading water, the contaminants on the sample surface were washed away.
According to our survey results, the majority of available acrylic polyols on the Vietnam market contain styrene components which are very susceptible to ultraviolet radiation. In our previous works [3, 6–8], photostabilizers were added in coating formulations to enhance the photostability of the coating. The obtained findings indicated that by incorporation of the UV absorber (at 2 wt% of TINUVIN 384) and HALS (at 1.5 wt% of TINUVIN 292) in the styrene acrylic polyurethane coating, its weathering resistance was enhanced significantly, but its mechanical property did not improved.
Pang et al [27] reported the effect of TiO2 nanoparticles on the degradation of acrylic polyurethane coatings during exposure to ultraviolet UV radiation. Recently, we reported the degradation of a water-borne acrylic coating and the influence of TiO2 nanoparticles on its photostability in accelerated weathering environment [1]. We also reported that epoxy coatings incorporated with TiO2 nanoparticles were successfully synthesized on the surface of steel substrates by solvent ultrasonication [19].
This work examined the influence of rutile TiO2 nanoparticles on the mechanical property, thermal stability, weathering durability and antibacterial property of the styrene acrylic polyurethane coating.
2. Materials and methods
2.1. Materials
HSU 1908 acrylic polyol solution (solid content of 55 wt%) was obtained from Industrial Resins Company A&P, Taiwan. The content of hydroxyl groups in this solid resin was 0.9 wt%.
Desmodur N-75 polyisocyanate solution (solid content of 75 wt%) was obtained from Bayer, Germany. The content of isocyanate groups in this solid resin was 17 wt%.
Rutile TiO2 (R-TiO2) nanoparticles, obtained from Sigma Aldrich, Singapore, had a mean diameter <100 nm and a specific surface area of 18 m2 g−1.
2.2. Samples preparation
R-TiO2 nanoparticles, which account for 0.25−5.0 wt% of the total solid of acrylic polyols and polyisocyanates, were added to the solutions of HSU.1908 acrylic polyols (HSU), followed by sonication (model TPC-15H, 35 kHz, Telsonic AG, Switzerland) for 3 h (called A component). Thereafter, the A component was mixed with the desmodur N.75 polyisocyanate (N75) with a 80:20 solid weight ratio under sonication stirring for 15 min.
For UV–visible and IR analysis, the coatings were prepared on teflon sheets. Whereas, they were coated on glass plates or steel substrates for measurements of the weight loss and mechanical properties, respectively. After 7 days of curing at room temperature, all the samples were dried in vacuum oven at 50 °C for 24 h before the analysis.
For antibacterial test, the neat and nanocomposite (with 2 wt% of R-TiO2 nanoparticles) coatings were coated on glass with dimensions of 5 × 5 cm2, then washed with acetone to remove all impurities on the surface, and autoclaved at 120 °C for 20 min just before use.
2.3. Characterization
2.3.1. Determination of the mechanical properties
The adhesion of coatings was evaluated by using the Elcometer Cross Hatch Cutter (England), according to the ISO 2409 standard, in which the adhesion was classified from the level 0 (the best adhesion) to the level 5 (the worst adhesion). The ball impact tests were performed by Impact Tester (model 304, Erichsen, Germany), according to the ISO 6272 standard.
2.3.2. Morphological analysis
The surface morphology of nanocomposite coating was studied using field emission scanning electron microscopy (FESEM). The coating surface was coated with a very thin carbon layer to avoid the charging effect caused by the nonconductive nature of coatings and to get high resolution image. The surface morphology was evaluated using a FE-SEM S4800 (Hitachi, Japan) system, which offers an ultra-high resolution at relatively low voltage.
2.3.3. Thermogravimetric analysis (TGA)
The thermogravimetry experiments used a well-equipped thermogravimetry analyzer (DTG-60H, Shimadzu, Japan), which was completely controlled by a PC computer. The samples (weight of ∼5.5 mg) were heated from room temperature to 800 °C at a rate of 10 °C min−1. Thermogravimetry experiments were conducted in air.
2.3.4. Accelerated weathering test
Accelerated weathering test was performed in a UV/condensation weathering chamber atlas UVCON UC-327-2 (USA) equipped with UVA-340 fluorescent lamps and operated under wet-cycle conditions of 8 h UV irradiation at 60 °C following by 4 h of dark water condensation (CON) at 50 °C (1 cycle consists 8 h UV and 4 h CON). The samples were dried in vacuum oven at 50 °C for 24 h before the analysis.
2.3.5. IR analysis
The chemical changes occurring upon the UVA/condensation accelerated weathering condition in exposed coatings were analyzed by Fourier transform infrared (FTIR) spectroscopy using NEXUS 670 from Nicolet (detector DTGS—KBr, resolution of 4 cm−1 and 256 scans). The variation of the following FTIR bands was monitored quantitatively through discrete measurements made on exactly the same spot of each sample, after various exposure times. From the decrease of the binder structural bands, the relative amount of remaining functional groups was determined by making the ratio of the IR absorbance at the corresponding wavenumber after a given exposure time (Dt) to the absorbance of the unexposed sample (D0) [1]:
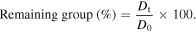
2.3.6. UV–vis analysis
An UV–vis spectrophotometer (GBC, CINTRA 40, USA) with 2 nm slip width and the transmission geometry was also employed to monitor the absorbance of the chromophores in the coatings with and without R-TiO2 nanoparticles. The samples used for UV–vis analysis were similar to that for FTIR analysis.
2.3.7. Weight loss of the coating
The samples to analyze the weight loss were prepared on glass plates with dimension of 100 × 70 × 2 mm3. The weight loss (Δmt) of the coatings after accelerated weathering test was determined by the difference between the weights of the samples (dried in vacuum oven at 60 °C until the constant weight) before (m0) and after (mt) the aging testing by the formula [1]:
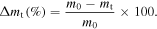
2.3.8. Antibacterial test of nanocomposite coating
E. coli DH5α was purchased from Invitrogen (USA). Luria-Broth medium was provided by Merck, Germany. To evaluate the cell density during culture, the instrument Beckman Coulter DU-730 (USA) was used. In this test, the optical density at a wavelength of 600 nm (OD600) measures the light absorbance of an E. coli cell culture sample. The OD600 value corresponds with the cell density or cell number in a given E. coli culture volume. Different cell strains may have different cell numbers at a given OD600 value, but OD600 = 1 usually means there are about 1 × 109 cells per ml culture.
Bacterial precultures were prepared with the aim of generating the subcultures of bacterial populations in a large scale in the lag phase so that the number of bacterial cells was constant before the log phase or exponential growth phase [28]. By this means, the growth rate of the bacterial cultures due to the presence of nanocomposites was evaluated over time. The OD600 values in the range 0.1–1.0 for cell densities of E. coli culture indicated the bacterial growth rates.
2.3.8.1. Precultures of bacteria
A volume of 100 μl of stock culture in glycerol of E. coli was pipetted into 3 ml of medium in a 15 ml test tube and shaken overnight at 200 rpm and 37 °C. Afterward, a 500 μl aliquot of preculture was inoculated into 100 ml of medium in a 500 ml Erlenmeyer flask and shaken at 200 rpm and 37 °C until the OD600 absorbance value reached 0.5. These precultures were used promptly for bacterial grow rate testing.
2.3.8.2. Monitoring of the bacterial lag–log growth phases in the presence of the neat and nanocomposite coatings
The monitoring test for the evaluation of the bacterial growth was adapted from the procedure in ASTM E 2149-10. The autoclaved coating samples were placed into the 100 ml bacterial preculture in a 500 ml erlenmeyer flask (as described previously) in which the OD600 had reached 0.5, and shaking was continued at 200 rpm and 37 °C. From this time forward, the OD600 value of each bacterial culture was monitored after 30 and 60 min, under the fluorescent tube ceiling lighting (36WT8 Philips). The obtained data were the average of three cultures.
3. Result and discussion
3.1. Effect of TiO2 nanoparticles on the mechanical properties of coating
For improvement of the mechanical properties, there are several studies on incorporating nanoparticles, especially TiO2 nanoparticles, into polymeric materials [14–21]. TiO2 nanoparticles have been found to improve the mechanical properties of the polar polymer (e.g. polyurethane [18]), more effective than that of the non-polar polymer (e.g. polypropylene, polyethylene [20, 21]). It could be explained by the presence of OH groups on the surface, TiO2 nanoparticles had a superior interaction with the polar polymer, compared with non-polar polymer.
Table 1 presents the values of impact strength and adhesion for the neat and nanomodified styrene acrylic polyurethane coatings. It can be seen from the table 1 that with the presence of R-TiO2 nanoparticles, the impact strength and adhesion of the coating were enhanced, with the 2 wt% content of the nanoparticles being the most effective.
Table 1. Values of impact strength and adhesion for the neat coating and coatings nanomodified with different content of R-TiO2 nanoparticles.
| Mechanical properties of the coating | ||
|---|---|---|
| Nano-TiO2 (%) | Impact resistance (kg cm) | Adhesion (level) |
| 0 | 120 | 1 |
| 0.25 | 125 | 1 |
| 0.5 | 130 | 1 |
| 1 | 140 | 0 |
| 2 | 145 | 0 |
| 5 | 140 | 1 |
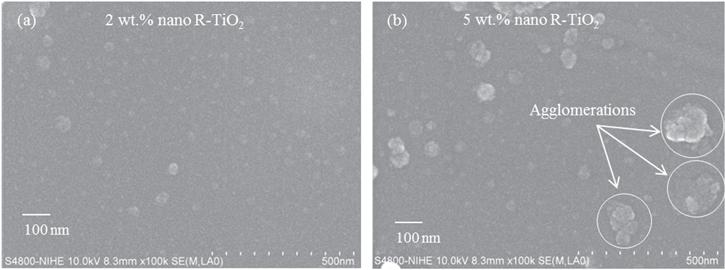
Appropriate dispersion of the nanofillers is very significant to take advantage of nanoscale reinforcement and to obtain desired properties. The small size of the nanoparticles is also advantageous as it enables their penetration into ultra-small holes, indentation and capillary areas in the polymer matrix. FESEM images of the nanocomposite coatings are shown in figure 1, with the contents of R-TiO2 nanoparticles of 2 wt% and 5 wt%, respectively. The coating modified by 2 wt% R-TiO2 nanoparticles showed no agglomeration of nanoparticle (figure 1(a)), whereas the one modified by 5 wt% R-TiO2 nanoparticles had some agglomeration of nanoparticles (figure 1(b)). The content of 2 wt% of nanoparticles were better dispersed in the coating matrix, verifying the better mechanical properties by dispersion of the impact force. Similar findings are also reported by other researchers [18, 20].
Figure 1. FE-SEM images of the nanocomposite coatings containing (a) 2 wt% and (b) 5 wt% R-TiO2 nanoparticles.
Download figure:
Standard image High-resolution imageThe average size of nanoparticles in the nanocomposite coating was approximately 40 nm, which indicated that the ultrasonic dispersion protocol was reliable to provide the nanocomposite coating.
The nanocomposite coating containing 2 wt% R-TiO2 nanoparticles was then also investigated by thermal, weathering and antibacterial tests.
3.2. Effect of R-TiO2 nanoparticles on the thermal stability of coating
Figure 2 shows TGA curves of the neat coating and its nanocomposites containing 2 wt% content of R-TiO2 nanoparticles. The thermal degradation parameters of the neat coating and nanocomposite coating were presented in table 2.
Figure 2. TGA curves of the neat and nanocomposite coatings.
Download figure:
Standard image High-resolution imageTable 2. Values of T5%, T50%, T75%, and remaining weight at 290 °C and 600 °C for the neat and nanocomposite coatings.
| Remaining weight (%) | |||||
|---|---|---|---|---|---|
| Coatings | T5% (°C) | T50% (°C) | T75% (°C) | At 290 °C | At 600 °C |
| Neat | 146.91 | 351.86 | 379.22 | 87.23 | 1.23 |
| Nanocomposite | 154.21 | 360.06 | 400.51 | 88.12 | 3.66 |
Note: T5% (°C), T50% (°C), T75% (°C) are temperatures at 5%, 50%, 75% loss in mass, respectively.
As shown in figure 2, thermal degradation proceeds via three overlapping mechanisms. The first, starting already around 150 °C, consists of homolytic scission of chemical bonds in the network, which influences the physical properties but does not cause a large weight loss. It can be assumed that the temperature at 5% loss in mass (T5%) is this initial thermal degradation temperature. For that temperature, it could be attributed to the release of adsorbed water and organic solvent in the coatings, cured at 146.9 °C and 154.21 °C for the neat and the nanocomposite, respectively. Thermal stability of these coatings was relatively poor, compared to those previously reported with the onset degradation temperature of around 240 °C–300 °C [29].
The first lower weight loss stage appeared from 150 °C to 290 °C. This weight loss could be attributed to the presence of impurities in coatings or to the dehydration (elimination of water molecules from the oxypropylene group –CH2–CH(OH)– and subsequent formation of double bonds [19]) or to the condensation of alcohol and cacboxylic acid functional groups on polymer chains.
The final thermal decomposition with higher weight loss occurred above 290 °C. This weight loss might be attributed to the scission of backbone chain in the urethane, acrylic and styrene segments [30, 31]. It can be assumed that the temperature at 50% loss in mass (T50%) is this final thermal degradation temperature. For that temperature, it occurred at 351.86 °C and 360.06 °C for the neat and nanocomposite coatings, respectively. As can be seen in figure 2, the 75% reduction of the initial weight of the samples cured at 379.22 °C and 400.51 °C for neat and nanocomposite coatings, respectively. At higher temperature, the char yields of the neat coating and its nanocomposite were found to be 1.23% and 3.66% at 600 °C, respectively.
3.3. Effect of R-TiO2 nanoparticles on the weathering resistance of coating
3.3.1. Effect of R-TiO2 nanoparticles on the structural stability of coating
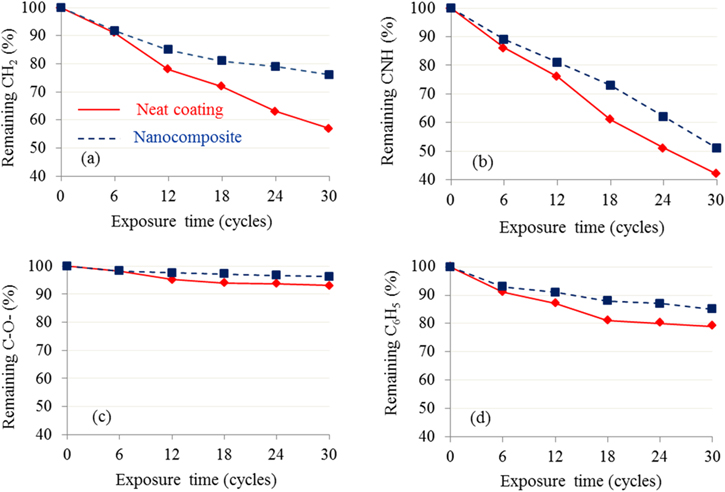
The changes in chemical structure of organic coatings due to degradation can be investigated by using infrared spectroscopy method. The weathering durability of coating can be evaluated from the chemical changes occurring upon the natural exposure or accelerated weathering test. The IR spectras of styrene acrylic polyurethane coatings unfilled and filled 2 wt% R-TiO2 nanoparticles before and after UV/CON accelerated weathering test (30 cycles) were represented in figure 3. The IR assignments for main characteristic bands of functional groups for these coatings are shown in table 3. A quantitative analysis for chemical changes after photo-degradation process, was carried out based on the IR characteristic bands of etylene (CH2), urethane (CNH), ester (C–O–) and benzyl (C6H5) groups, which located at 2935, 1525, 1150 and 760 cm−1, respectively, and was represented in figure 4.
Figure 3. Infrared spectra of neat coating and coating containing 2 wt% R-TiO2 nanoparticles before and after 30 cycles of accelerated weathering test.
Download figure:
Standard image High-resolution imageTable 3. The IR assignments for the functional groups of neat coating and coating containing 2 wt% R-TiO2 nanoparticles after UV/CON accelerated weathering test.
| Observations | |||
|---|---|---|---|
| IR band (cm−1) | Characteristic functional group | Intensity | Changes |
| 3480 | O–H stretching | Weak | Increased |
| 2935 | C–H stretching in CH2 | Strong | Significantly decreased |
| 1730 | C=O stretching in ester | Strong | Unclearly changed |
| 1525 | C=NH stretching in urethane | Strong | Significantly decreased |
| 1150 | C–O– stretching in ester | Strong | Slightly decreased |
| 760 | C–H stretching in C6H5 | Weak | Significantly decreased |
Figure 4. Chemical change after accelerated weathering test for the neat and nanocomposite coatings.
Download figure:
Standard image High-resolution imageAs can be seen in figure 4 during the aging process, the intensity bands assigning to the CH2, CNH, C–O– and C6H5 groups decreased. As observed in figure 3, the presence of R-TiO2 nanoparticles led also to slow down the formation of oxidative products (OH, COOH) during the aging process. Thus, R-TiO2 nanoparticles mitigated the degradation of the coating.
Degradation mechanism of the acrylic polyurethane coatings had been proposed in literature [3, 4]. During the accelerated weathering test, the coatings might be simultaneously affected by various factors including ultraviolet radiations, heat, moisture and oxygen. The ultraviolet radiation may cause the polymer chain scission, then with the presence of oxygen, the photooxidation of polymer would take place. Besides, the coating might be also deteriorated by moisture and water, via the hydrolysis reaction of the hydrophilic ester and urethane groups in polymer chains. High temperature might also accelerate this process. These degradation mechanisms should be related with the modifications of IR bands characterizing for the CH2, CNH, C–O–, C6H5–, OH and COOH groups, which were previously mentioned.
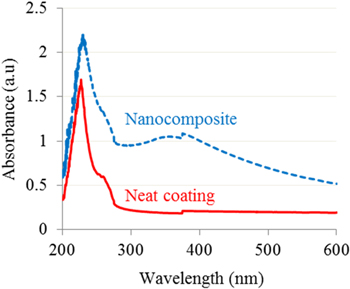
This can be attributed by the fact that, the R-TiO2 nanoparticles may be absorbed the light radiation in UV–vis region ranging from 200 to 400 nm. When R-TiO2 nanoparticles absorb an amount of energy of UV-light, an electron will jump from the forbidden band to conduction band resulting in the production of an electron–hole pair. Then these positive hole and electron can attack the water, oxygen molecules and hydroxyl groups on the surface of R-TiO2 nanoparticles leading to the formation of free radicals. Those radicals then initiate the photocatalytic reactions and hence might degrade polymers. Thus, the UV absorption of R-TiO2 nanoparticles always induces two opposite effects: UV shielding and photo-degradation for polymer substrate [1, 17, 22, 32]. To explain the mechanism of UV shielding of the nanoparticles, UV–vis spectra of neat coating and its nanocomposite with 2 wt% R-TiO2 nanoparticles is shown in figure 5. It can see that the UV absorbance of the coatings was strong. The UV absorption of R-TiO2 nanoparticles caused the UV light shielding effect for polymer coating and the coating reinforced by nanoparticles have a better resistance to aging conditions compared to that of neat polymer.
Figure 5. UV–vis spectra of the neat and nanocomposite coatings.
Download figure:
Standard image High-resolution image3.3.2. Weight loss of the coating
The coating degradation upon weathering test can be evaluated by various physical methods, such as discoloration, loss of gloss, chalking, cracking, thickness decrease and weight loss. The average loss of 5−25 μm per year in the dry film thickness for polymers in the natural exposure was reported in [33]. In our previous works [1], we reported the weight loss of a water-borne acrylic coating with and without R-TiO2 nanoparticles were 19.2 and 28% after 96 cycles of UV-B/CON aging, respectively.
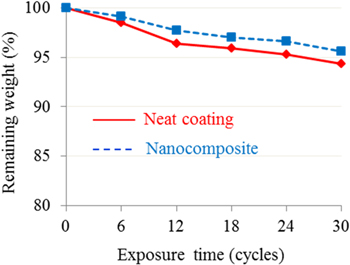
Figure 6 presents the weight loss data for neat styrene acrylic polyurethane coating and its nanocomposite during 30 cycles of accelerated weathering test. As shown in this figure, the nanocomposite coating exhibited lower weight loss compared to that of the neat coating. After 30 cycles of UV-A/CON aging, the weight losses were 5.7 and 4.4 wt% for the neat coating and its nanocomposite, respectively.
Figure 6. Influence of nano-TiO2 on weight loss of the coating during UV-A/CON aging.
Download figure:
Standard image High-resolution imageDuring accelerated weathering test, two competing mechanisms may be involved: (I) photooxidation where oxygen atoms are bonded to the polymer matrix, thus leading to an increase of the sample weight; (II) scissions of the polymer chain by photolysis, hydrolysis and photocatalytic reactions which produce low molecular weight oxidation products, such as CO2, CH4, aldehydes, acids, which can either evaporate or be washed away by moisture, thus leading to the weight loss of coating [1, 16, 34]. In this study, the mechanism (II) appears to be dominant.
3.3.3. Impact strength and adhesion measurements
Table 4 presents the impact strength and adhesion of neat coating and its nanocomposite coating before and after 30 cycles of accelerated weathering test. It can be seen from the table 4 that after 30 cycles, impact strength and adhesion of neat coating strongly decreased. For the nanocomposite coating, after 30 cycles, its adhesion did not change, whereas its impact strength slightly reduced from 145 to 130 kg cm. Thus, R-TiO2 nanoparticles mitigated the mechanical degradation of the coating.
Table 4. Impact strength and adhesion of the neat coating and nanocomposite coating before and after 30 cycles of accelerated weathering test.
| Impact strength (kg cm) | Adhesion (level) | |||
|---|---|---|---|---|
| Coating | Before | After 30 cycles | Before | After 30 cycles |
| Neat coating | 120 | 60 | 1 | 2 |
| Nanocomposite | 145 | 130 | 0 | 0 |
3.4. Effect of nanocomposite coating on the growth rate of the bacterial cultures
Figure 7 shows the influence of the nanocomposite coating (2 wt% R-TiO2 nanoparticles) on the growth rate of the E. coli liquid culture, during 60 min since its presence in the liquid culture.
Figure 7. Influence of the nanocomposite coating on the growth rate of the E. coli liquid cultures.
Download figure:
Standard image High-resolution imageAs seen in figure 7, the bacterial concentrations of mixed culture with the nanocomposite coating were lower than that of neat coating. The bactericidal efficiency reached 6.1% after 60 min. Thus, these data indicate that R-TiO2 nanoparticles interfered with the growth and reproduction of bacteria via photocatalytic effect. A possible explanation for this low bactericidal efficiency is the very low dose daylight source from the fluorescent tube ceiling lighting. In order to avoid damage by UV irradiation alone, the fluorescent tubes were used with illumination of ∼200 lux (<4 W m−2). As in available literatures, by using the relatively strong UV light intensity with photon wavelengths usually between 300 nm and 400 nm (∼10 W m−2 and up to 500 W m−2), other researches had reported the effect of photocatalytic TiO2 nanoparticles on microorganisms [35–37]. At room temperature, R-TiO2 nanoparticles had a band gap of ∼3.0 eV, that could absorb the energy amount of photons from UV-light. Ultraviolet photons associated with the 254 nm UV light have quantum energies of 4.9 eV, whereas the energy range for the visible photons was only from 1.6 to 3.1 eV. On other hand, in the log phase, the doubling in number of new bacteria should continue at a constant rate, which could dominate the photocatalytic effect of R-TiO2 nanoparticles.
These results indicated that the E. coli bacterial growth was slightly restricted in the presence of nanocomposite coating. The higher bactericidal efficiencies on bacteria were obtained by using UV radiation, such as around 3 log after 30 min [38] and 6 log after 90 min [39]. Verdier et al [25] had reported the lower antibacterial activity against E. coli for acrylic/TiO2 nanocomposite coatings under lower UV light intensity (2.5 W m−2). In their work, the E. coli bacterial reduction reached only 0.9 log after 120 min of radiation.
4. Conclusion
The main findings of this study showed that homogeneous styrene acrylic polyurethane coatings containing R-TiO2 nanoparticles (0.25–5.0 wt%) were successfully synthesized by using ultrasonication; the mechanical tests indicated that incorporation of R-TiO2 nanoparticles improved the impact strength and adhesion of coating, with the content of 2 wt% being the most effective; the FESEM images revealed that R-TiO2 nanoparticles was apparently dispersed homogeneously in a coating matrix (at 2 wt%). The average size of nanoparticles in the nanocomposite coating was approximately 40 nm. The TGA test showed that with the presence of R-TiO2 nanoparticles, the decomposition temperature of coating increased from 146.9 °C to 154.21 °C. For the temperature at 50% loss in mass (T50%), it was found that the T50% of the neat coating is 351.86 °C. Adding the 2 wt% R-TiO2 nanoparticles into coating increased the T50% value to 360.06 °C. Data analysis from accelerated weathering test indicated that R-TiO2 nanoparticles mitigated the chemical change, weight loss and mechanical degradation of the coating. The antibacterial test showed that in the nanocomposite coating, R-TiO2 nanoparticles exhibited their photocatalytic effect in the inhibition against E.coli bacterial growth.
These findings indicated that not only as nanofiller in polymer matrix, R-TiO2 nanoparticles also play a both two other roles, such as the photostabilizer and the photocatalysts. The effects of R-TiO2 nanoparticles on the self-cleaning activity of coating are now under investigation.
Acknowledgments
This work is financially supported by Vietnam Academy of Science and Technology's Foundation for Material Science (funding grant number VAST03.05/14-15). The authors would like to thank Acad. Nguyen Van Hieu for his valuable comments throughout the course of this project.