Abstract
The present scientific endeavour focuses on the optimization of process parameters using central composite design towards development of an efficient technique for the biosynthesis of silver nanoparticles. The combined effects of three process variables (days of fermentation, duration of incubation, concentration of AgNO3) upon extracellular biological synthesis of silver nanoparticles (AgNPs) by Aspergillus wentii NCIM 667 were studied. A single absorption peak at 455 nm confirming the presence of silver nanoparticles was observed in the UV-visible spectrophotometric graph. Using Fourier transform infrared spectroscopic analysis the presence of proteins as viable reducing agents for the formation AgNPs was recorded. High resolution transmission electron microscopy showed the realization of spherically shaped AgNPs of size 15–40 nm. Biologically formed AgNPs revealed higher antimicrobial activity against gram-negative than gram-positive bacterial strains. We present the enumeration of the properties of biosynthesized nanoparticles which exhibit photocatalysis exhausting an organic dye, the methyl orange, upon exposure to sunlight thereby accomplishing the degradation of almost (88%) the methyl orange dye within 5 h.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 3.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
One of the most sophisticated branches of modern day science is nanotechnology which involves the fabrication, management and usage of materials in the nanosize range [1]. The fabrication of silver nanoparticles (AgNPs) has shot up in the present day world (in both its metallic and ionic forms), possessing properties like cytotoxic, anti-fungal, anti-inflammatory, antiangiogenic and anti-permeability [2]. Silver nanoparticles possess the potentialities to be applied in innumerable fields such as antimicrobial factor in food to increase their shelf-life and also in textiles. Development of resistant microbial strains is a mounting apprehension among researchers leading to the onset of modern day research involving metallic nanoparticles due to their excellent capability of exhibiting antimicrobial activity [3].
Environmental aspects are the most crucial of all factors when it comes to implications of any experimental procedures. Similarly even in case of nanoparticle synthesis, chemical routes brings about the usage of toxic reducing agents giving rise to detrimental effects threatening the well-being of human health as well as the environment. This scenario creates an emergence in developing synthesis techniques, which would be inexpensive, greener and larger-scale. Present day nanotechnology research accepts the challenge and aims at developing toxicity-free synthesis of metal nanoparticles. The main steps for green synthesis of AgNPs' involving safe chemical methods are (i) identification of potential solvent medium and (ii) making choice of environmentally compatible reducing agent. The formation of Ag0 by reducing Ag+ ions along with various complexes leads to the agglomeration of Ag0 and these complexes, thereby forming colloidal silver nanoparticles (AgNPs). The energy band obtained from the absorption spectrum is recognized to be due to the combined excitation of the electron gas present within the particles in addition to the intermittent alteration in electron density occurring on the particle surface [4].
Biological route of nanoparticle synthesis which involves the usage of microorganisms has already been established to be an efficient methodology. The microbial synthesis of nanoparticle is categories into (i) intracellular (ii) extracellular in accordance with the site of nanoparticle formation. Fungal strains were found to be more advantageous compared to other microorganisms AgNPs synthesis. Fungal mycelium possesses the competency to sustain high flow pressure and agitation making it quite stress-free in managing cultivation of fungal strains in a bioreactor. By the virtue of their ability to secrete vast number of substances with reduction properties fungal strains produce nanoparticles extracellularly. Among them, Fusarium oxyporum, Aspergillus niger, and Aspergillus fumigatus were the most studied strains due to their calibre to secrete extracellularly. The reductive proteins are secreted extracellularly and react with the precursor thereby leading to the synthesis of AgNPs; hence reducing the labour in downstream processing [5–7]. Recently biological synthesis of silver nanoparticles was accomplished using carambola fruit extract which led to the rapid reduction of silver ion [8]. Kumar et al [9] described successfully production of nanoparticles which are composed of silver and silver chloride nanoparticles giving rise to a photocatalyst which effectively degraded an organic dye called rhodamine upon reduction by leaf extract of Solidago altissima (S. altissima) They formulated the greens synthesis procedure for the production of the effective plasmonic photocatalyst in two-steps the first being the fabrication of AgCl NPs upon reduction of AgNO3 by aqueous leaf extract of S. altissima, and the second being Ag and AgCl NPs formation through photoreduction of AgCl NPs [9]. A recent article reported that Chlorella vulgaris a microalga which secrets carbohydrates are in turn being employed for the reduction of silver nitrate and capping silver nanoparticles [10].
The variables of the experimental methods have been optimized by keeping all the autonomous elements settled aside from one which is changed. Burdens of single variable streamlining strategies energize [11] the appointing of reaction surface system which edifies the mix impacts of all the exploratory components of an experimental set up [12]. Response surface methodology is a measurable practice which is standard on the irreplaceable standards of dimensions, randomization and repetition which condenses the procedure of improvement by contemplating the common associations among the variables over a scope of qualities giving it factual legitimacy. Notwithstanding investigating the impacts of the autonomous variables, this test technique produces a numerical model that decisively portrays the general procedure. Inferable from its operative effectiveness, response surface methodology is currently being routinely utilized for improvement of broadly different frameworks [13–15]. Among the different response surface methodologies, it was observed that central composite design has been used for the last enhancement of sought procedure alongside the Box–Behnken design, and Doehlert matrix.
An extremely regular azo dye, methyl orange (MO) finds widespread usage in the textile, foodstuff, paper, and leather industrial arena. It is genuinely a natural concern which emerges because of the arrival of MO and its items in the earth contributing to significant contamination. The photocatalytic treatment of wastes containing dyes has been a genuine test for the scientific fraternity and has been a road for exploratory investigation for a long time prompting accumulation of published reports. Presently biogenic nanoparticles find considerable utilization in successful and enhanced strategy for degradation and elimination of these hazardous natural dyes. Metallic nanoparticles have extraordinary properties like photocatalytic elimination of hazardous organic compounds under the exposure of solar illumination and at encompassing temperature. In the present scientific endeavour efforts were made to assess the degradation of methyl orange dye to discover an answer towards natural [16–19].
This paper encompasses the optimization of duration of fermentation, hours of incubation and volume of silver nitrate, the important process parameters in the experimental methodology involving a fungal strain, Aspergillus wentii NCIM 667 for the production of silver nanoparticles. This work is thought to be the first ever report which focuses on the extracellular biological production of silver using a strain of Aspergillus wentii.
2. Materials and methods
2.1. Microorganism and cultural condition
Aspergillus wentii NCIM 667 was bought from National Chemical Laboratory, Pune, India. It was maintained on potato dextrose agar medium. The media which was used for the biosynthesis of silver nanoparticle typically contained of MgSO4.7H2O of 0.5 g l−1, KH2PO4 of 1 g l−1, NH4NO3 of 3 g l−1, yeast extract-1 and cane molasses-140 (wet weight), pH was adjusted to 6. Prior use molasses (140 g) was treated with 35 ml l−1 of 1 N H2SO4 to reduce its sugar content to about 25% sugar level. In practice, the molasses suspension was kept in a boiling water bath for an hour, cooled and neutralized with lime water. It was kept at room temperature over night and the clear supernatant was decanted. Requisite amounts of other ingredients were then added, pH was adjusted and volume was made up; total sugar content of the medium was estimated to be 15% (w/v). 7-days old potato dextrose agar slants were used for harvesting spores followed by suspension of the same in 10 ml 0.1% (v/v) tween 20 solution. Finally employing a Neubauer chamber the spores were counted under a light microscope. Suitably diluted 1 ml of suspension containing 2 × 107 spores was mixed to 100 ml liquid medium in a 500 ml conical flask. A rotary incubator shaker with internal temperature set at 30 °C was used for placing the flasks for a scheduled period to allow growth; thereafter the mycelia were separated from the culture filtrate which was preserved at 4 °C until used [20].
2.2. Extracellular synthesis of AgNPs
Typically a stock solution of silver nitrate was prepared by dissolving 0. 84 g silver nitrate in 100 ml deionized water. For the biosynthesis of AgNPs to occur 100 ml 50 mM AgNO3 solution was mixed with 100 ml fungal culture filtrate to obtain a solution mixture where the final concentration of silver nitrate becomes 25 mM thereafter the solution was incubated (dark condition) for 36 h at 30 °C. A control was maintained having only the culture filtrate and devoid of AgNO3.
2.3. Design of experiments and optimization
This study focuses on the effect of three process parameters using central composite design (CCD), which are as follows: days of fermentation (A), duration of incubation (B) (mixture of culture filtrate and silver nitrate) and volume of silver nitrate (C) on the biosynthesis of silver nanoparticles thereby optimizing the same. Experimental factors considered for the design was put to test at three different levels (−1, 0, 1). The design comprising of 30 trials was constructed using design expert 9.0.5.1 shown in table 1. The experimental methodology was framed in accordance with the design where tests were executed with 250 ml erlenmeyer flask containing culture filtrate and silver nitrate solution. The flasks were incubated at 30 °C for a maximum of 36 h to elucidate the consequence of time. Discrete sets of samples were withdrawn at a fixed time interval. The entire experiment was bnanoparticles was recorded and was designated as the dependent variable or response (Y). Response surface regression technique was employed to fit the experimental results of CCD using the following second order polynomial equation:
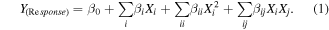
Table 1. Experimental matrix and responses for CCD.
| Run | Factor 1 | Factor 2 | Factor 3 | Response | |
|---|---|---|---|---|---|
| A (day) | B (hour) | C (ml) | Absorbance at 455 nm | ||
| Actual | Predicted | ||||
| 1 | 14 | 24 | 15 | 0.57 | 0.573 |
| 2 | 14 | 24 | 15 | 0.57 | 0.573 |
| 3 | 21 | 36 | 25 | 0.7 | 0.718 |
| 4 | 14 | 12 | 15 | 0.54 | 0.539 |
| 5 | 21 | 12 | 5 | 0.42 | 0.445 |
| 6 | 14 | 24 | 5 | 0.4 | 0.397 |
| 7 | 14 | 24 | 15 | 0.57 | 0.537 |
| 8 | 21 | 12 | 25 | 0.64 | 0.64 |
| 9 | 21 | 36 | 5 | 0.58 | 0.581 |
| 10 | 14 | 36 | 15 | 0.62 | 0.61 |
| 11 | 7 | 36 | 25 | 0.5 | 0.504 |
| 12 | 14 | 24 | 15 | 0.57 | 0.573 |
| 13 | 21 | 24 | 15 | 0.67 | 0.667 |
| 14 | 14 | 24 | 25 | 0.59 | 0.583 |
| 15 | 7 | 36 | 5 | 0.35 | 0.352 |
| 16 | 14 | 24 | 15 | 0.57 | 0.573 |
| 17 | 7 | 12 | 25 | 0.52 | 0.521 |
| 18 | 14 | 24 | 15 | 0.57 | 0.573 |
| 19 | 7 | 24 | 15 | 0.5 | 0.493 |
| 20 | 7 | 12 | 5 | 0.27 | 0.27 |
A is days of fermentation, B is duration of incubation, C is volume of silver nitrate
Typically in the equation (1) Y represents the predicted response, β0 denotes the regression coefficients, βi is the linear coefficient, βii signifies the quadratic coefficients while βij is the interaction coefficients and Xi (i = 1, 2, 3) represents the coded level of independent variables. In the present context the independent variables were decided to be coded as A, B and C therefore the second order polynomial equation was untaken to be as follows:
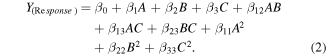
2.4. Characterization of silver nanoparticles
A Perkin Elmer UV-visible spectrophotometer within the range 300 and 800 nm wavelength was used for the spectroscopic examination of the colloidal mixture. Dried powder of AgNPs was exposed to x-ray diffraction analysis using a Rigaku Ultima-III x-ray diffractometer (operating voltage 40 kV, Cu-Kα radiation with λ = 0.154 nm). Fourier transform infrared (FTIR) spectroscopy analysis of the silver nanoparticles was accomplished using IR-prestige Fourier transform infrared spectroscope (Shimadzu, Japan). The AgNPs solution (powered AgNPs were mixed with deionised water) having concentration of 40 μg ml−1 was prepared for high resolution transmission electron microscopy (HRTEM) and dynamic light scattering analysis. By the help of a dynamic light scattering analyser (ZEN 1600 Malvern, USA) the typical size of biologically produced AgNPs was recorded. The samples were scanned by JEOL-2010 high resolution TEM (operating voltage 200 kV) to observe the shape and size of these Ag nanoparticles.
2.5. Antimicrobial activity of silver nanoparticles
Two strains of bacteria (S. aureus ATCC 25923 and E. coli wild type) and two multidrug resistance strains of bacteria (K. pneumonia and P. aeruginosa) were considered for the antimicrobial activity test. Typically the experiments involved mixing of 25 μl nanoparticle in solution with 65 μl of fresh Mueller–Hinton broth followed by serial double-dilution into eight microwells. Thereafter each well of the 96-well microtiter plates was filled with 10 μl of the bacterial culture broth. Finally optical density measurements where carried out at 620 nm upon incubation for 0 and 6 h [21].
2.6. Photocatalytic degradation
The photocatalytic degradation of methyl orange was assessed by biologically synthesized AgNPs. The whole test method was conveyed in the open-air with sun as the principle origin of light. A colloidal suspension was prepared upon mixing 20 mg of AgNPs to 50 ml of methyl orange solution. To affirm uniformity of AgNPs throughout the suspension, it was stirred for roughly 30 min in dark. The suspension was kept under daylight into a glass measuring glass at the time of the reaction. Examinations were completed on a sunny day at Johannesburg city, South Africa between 11 a.m. and 4 p.m. (climatic temperature 25 °C–27 °C). The absorption spectrum of the suspension was measured intermittently utilizing an UV–visible spectrophotometer after centrifugation to guarantee the degradation of methyl orange solution [16–19].
3. Result and discussions
3.1. Biosynthesis of nanoparticle
Production of nanoparticles was ascertained by recording the absorbance of the colloidal suspension using UV-visible spectrophotometry within the range of 300–600 nm. An absorption peak at 455 nm as shown in figure 1 corroborates to the specific surface plasmon resonance (SPR) spectra of silver nanoparticles produced by the culture filtrate of Aspergillus wentii indicating the presence of AgNPs. Surface plasmon resonance is the phenomenon which primarily controls the absorption spectra generated, confirming the occurrence of metal nanoparticles. The SPR peak of AgNPs in aqueous solution modifies to longer wavelengths as the particle size grow. The phenomenon of plasmon absorption by the assembly of silver nanoparticles attributing to their position and shape depends inevitably on size of the nanoparticles, molecules responsible for stabilization or particles which got adsorbed onto the surface, in addition to the dielectric constant of the reduction medium [5]. In accordance with the Mie's theory [22], the absorption spectra exhibited a single surface plasmon resonance band generated due to the presence of spherically shaped nanoparticles leading to the decrease in symmetry of the nanoparticle as result of the increase in number of SPR peaks [5]. The reaction mixture of the present study exhibits a single surface plasmon resonance band at 455 nm uncovering spherically shaped of AgNPs, which was further settled by TEM pictures.
Figure 1. UV-Vis spectra of synthesised AgNPs.
Download figure:
Standard image High-resolution imageIt is still a less known actuality that why AgNPs are best shaped in the absence of light. By the virtue of biologically initiated reduction to form metallic silver, which is attributed to the synergistic effects of the DNA, protein containing sulphur as one of the structural component and reduced nicotinamide adenine dinucleotide (NADH)-dependent nitrate reductase. The factor which is of prime importance in the biosynthesis process is NADH-dependent reductase. The NADH to oxydized nicotinamide adenine dinucleotide (NAD+) oxidation reaction is catalysed by the reductase enzyme, followed by oxidation of the enzyme and accomplishment of metal ion reduction. The silver ions get reduced to metallic upon induction of reductase enzyme by the nitrate ions [7].
The low wavelength region UV-Vis spectrum of the reaction mixture at the end of 36 h is shown in the inset of figure 1. A prominent peak at 276 nm is visible and is due to the presence of aromatic amino acids of proteins. The tryptophan and tyrosine residues of proteins undergoes electronic excitation giving rise to the absorption band at 276 nm which is quite an established fact [23], signifying proteins discharge into the medium as a result of the metabolic activity of A. wentii and recommends a conceivable mechanism which brings about the reduction of the metal ions when acted upon by the culture filtrate solution. The proteins could most likely have impact in shaping an external surface covering on the metal nanoparticles which at the end counteracts the particle agglomeration and would have aided in balancing out the same in the medium.
3.2. Optimization of silver nanoparticles biosynthesis using response surface methodology
Thirty experiments involving different days of fermentation (A), duration of incubation (B) and volume of silver nitrate (C) were completed, and the after effects of the trials are exhibited in table 1. Higher production of silver nanoparticles (>0.5) was demonstrated in the treatment runs 3, 8, and 13 while run number 3 was the highest among them (0.7), which was accomplished under the conditions of 21 days of fermentation, 36 h of incubation and 25 ml of AgNO3. Multiple regression investigation of the experimental findings was computed, which headed to the establishment of a second-order polynomial model, which defines the part played by each of the variables and their second-order associations with the biosynthesis of the silver nanoparticles. The R2 values connote the inconsistency in the experimental response values, clarified by the experimental factors and their collaborations. Strength of the model is determined by the fact that by what extent is the numerical value of R2 closer to 1, which was 0.9987 in case of the present model signifying elucidation by the model, can be made on the response inconsistencies to an extent of 99.87%. A well fitted relationship between the experimental and predicted response values was established on the basis of the R2 value indicating appropriateness of the model for biosynthesis of AgNPs. The calculated adjusted R2 value of 0.9975 is also a clear indication towards a considerably significant model which brought about a reasonably well-thought-out analysis of the response trend. In order to elucidate the significance as well as the adequacy of the model the F, P and sum of square values were computed and are shown in table 2. Estimation of the model coefficients were accomplished through the usage of multiple regression investigation. The significance and adequacy of the model with help of analysis of variance (ANOVA) through Fisher's statistical analysis are listed in table 3. The value of p < 0.0001 for the AgNPs synthesis recommended that statistically significant of the model and on the basis of the p-value the model f-value was calculated to be 848.04, whose probability of existence owing to noise was 0.01%. The statistical values of ANOVA given in table 3 indicate that the model terms are significant with a confidence interval of 95%. On the other hand, the values, which are greater than 0.10, were considered insignificant. A, B, C, AB, BC and C2 are significant model terms making them the limiting factors which, majorly contributes to the appreciation and depreciation of the production rate. To assess the association between the dependent and the independent variables, and to obtain the highest amount of biologically produced silver nanoparticles corresponding to the optimum levels of days of fermentation (A), duration of incubation (B) (mixture of culture filtrate and silver nitrate) and volume of silver nitrate (C), a second-order polynomial model was anticipated to ascertain the optimum levels of these variables. By applying multiple regression analysis to experimental data, the second-order polynomial equation that defines the predicted response (Y) in terms of the independent variables (A, B and C) was obtained:
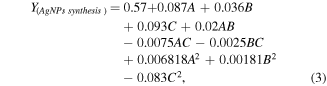
where Y is the response, A is the coded value of days of fermentation, B is the coded value of duration of incubation, C is the coded value of volume of silver nitrate.
Table 2. Estimated values for the optimization of biosynthesized silver nanoparticles using central composite design.
| Source | Sum of squares | f-value | p-value |
|---|---|---|---|
| Model | 0.21 | 848.04 | <0.0001 |
| A | 0.76 × 10−1 | 2694.47 | <0.0001 |
| B | 0.13 × 10−1 | 461.36 | <0.0001 |
| C | 0.86 × 10−1 | 3078.93 | <0.0001 |
| AB | 0.32 × 10−2 | 113.92 | <0.0001 |
| AC | 0.45 × 10−3 | 16.02 | 0.0025 |
| BC | 0.5 × 10−2 | 177.99 | <0.0001 |
| A2 | 0.1278 × 10−3 | 4.55 | 0.0587 |
| B2 | 0.9091 × 10−5 | 0.32 | 0.5820 |
| C2 | 0.19 × 10−1 | 677.37 | <0.0001 |
| Residual | 0.2809 × 10−3 | ||
| Lack of fit | 0.2809 × 10−3 | ||
| Pure error | 0.00 | ||
| Corrected total sum of squares | 0.21 |
A is days of fermentation, B is duration of incubation(hour), C is volume of silver nitrate (ml)
Table 3. Statistical values of ANOVA.
| Standard deviation | 0.0053 |
| Mean | 0.54 |
| Coefficient of variation (%) | 0.99 |
| R-squared | 0.9987 |
| Adjusted R-squared | 0.9975 |
| Predicted R-squared | 0.9935 |
| Adequate precision | 115.27 |
It is a significant vital undertaking to check the fitted model to affirm that it offers an acceptable approximation with the actual framework. Analysis and optimization of the fitted model would be erroneous if the model does not show an adequate fit. Figure 2(a) shows the normal probability plot of the residuals which reveals the systematic deviations from the expectations. The residuals plot where the residuals are plotted against the normal values of the model depicts that the points are nearby to a diagonal line which implies that the errors are normally dispersed and are individually independently depicting a homogenous error variances indicating a good fitted model. Residuals from the fitted model are normally distributed therefore all the major assumptions of the model have been validated. The plot shown in figure 2(b) depicts an agreeable correlation between the predicted and actual values of responses.
Figure 2. (a) Normal plot of residuals and (b) predicted- versus measured- absorbance for biosynthesis of AgNPs model.
Download figure:
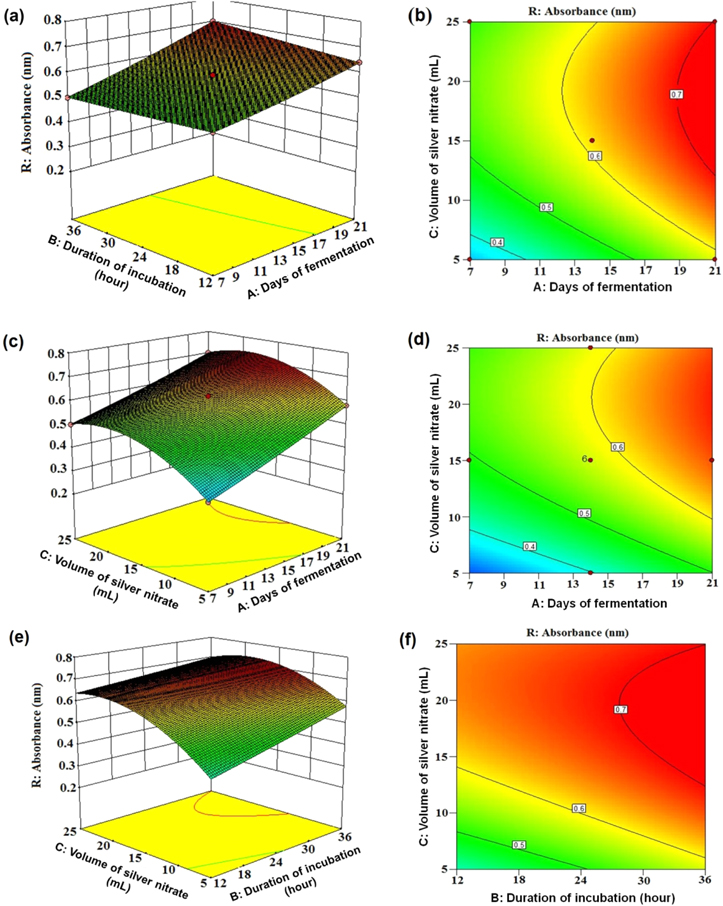
Standard image High-resolution imageFigure 3 shows the interaction effects among the process parameters and their optimal levels elucidated through the 3D surface and their corresponding contour plots sustaining one of the parameters static at the optimum value while the other two are permitted to fluctuate. Figures 3(a) and (b) show interaction between days of fermentation and incubation time. Figures 3(c) and (d) represent the interaction between volume of silver nitrate solution and days of fermentation. Interaction between the volume of silver nitrate and the incubation time was depicted in figures 3(e) and (f). From the plots it is quite clear that there is a profound effect of all the three experimental parameters on the production of silver nanoparticles. It is also most evident that the interaction between the volume of silver nitrate with time of incubation (figures 3(e) and (f)) is much stronger than the other two (attributed to the characteristic curve nature of the plotted lines and highest f value of 177.99 as shown in table 2).
Figure 3. Surface plots of AgNP biosynthesis absorbance (nm) versus: (a) duration of incubation (hours) and days of fermentation, (c) volume of silver nitrate (ml) and days of fermentation, (e) volume of silver nitrate and duration of incubation. Contour plot of AgNP biosynthesis absorbance (nm) versus: (b) duration of incubation and days of fermentation, (d) volume of silver nitrate and days of fermentation, (f) volume of silver nitrate and duration of incubation.
Download figure:
Standard image High-resolution image3.3. Validation of the statistical model
Keeping in mind the end goal to reinforce the importance of the models for foreseeing the maximized production of AgNPs utilizing culture filtrate of Aspergillus wentii NCIM 667, three validation runs were executed by means of the optimum conditions; the results are listed in table 4. The observed AgNPs absorbance value was 0.7 and the predicted value from the polynomial model was 0.718 with the aim of validating the model under the tested conditions. The optimal levels of the process parameters for biosynthesis of AgNPs by A. wentii NCIM 667 were 21 days of fermentation, 36 h of incubation and 25 ml AgNO3 solution. The good agreement between the anticipated and the trial values attests that response surface methodology (RSM) with the measurable outline of examinations could be effectively used to streamline the experimental parameters and to assess the significance of individual and intuitive impacts of the test variables in the generation of AgNPs. It is an event of RSM that could be beneficially drawn in to process enhancement.
Table 4. Validation experiments.
| Number of run | Absorbance at 455 nm |
|---|---|
| 1 | 0.75 |
| 2 | 0.63 |
| 3 | 0.72 |
| Average | 0.7 |
| Predicted | 0.718 |
3.4. XRD analysis
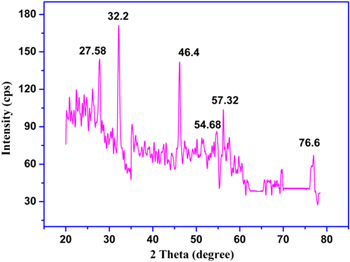
Crystallinity and different phases as obtained from the XRD analysis of the synthesized biogenic nanoparticles were identified and have been depicted in figure 4, which consists of six peaks at 27.58°, 32.2°, 46.4°, 54.68°, 57.32° and 76.8° corresponding to (220), (122), (231), (331), (241) and (311) planes of Ag, respectively (as correlated to JCPDS: File No. 4-783). The nanoparticles are crystalline and face-centered cubic in nature found in [17, 24].
Figure 4. XRD pattern of the AgNPs.
Download figure:
Standard image High-resolution image3.5. FTIR spectroscopy
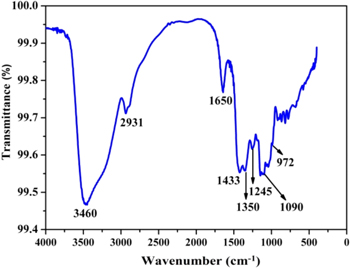
For the recognition of the molecules accountable for reduction and stabilization of Ag nanoparticles Fourier transform infrared spectroscopy was exploited and the corresponding FTIR spectrum obtained in the transmittance mode is shown in figure 5. The bands observed at 1650 and 2931 cm−1 denote the C=O stretching of tertiary amides and C–H stretching of aldehydes, respectively. The bands obtained at 1245 and 3460 cm−1 correspond to the C–O stretching and O–H stretching of benzene ring compounds (like organic acid), respectively. Two bands at 1350 cm−1 and 1090 cm−1 indicate the C–N stretching vibration of aromatic and aliphatic amines, respectively [25, 26]. The C–H bending for alkenes and alkanes were identified by the presence of the band at 972 cm−1 [17]. Amino acid residues and peptides containing carbonyl groups of have strong capability to bind to silver [17, 23, 27]. The FTIR spectrum of the silver nanoparticle and the fungus culture filtrate reaction mixture exposed the presence of two bands at 1650 and 1433 cm−1 in which band at 1650 cm−1 is recognized as the amide vibrations leading to carbonyl stretch and amide linkages of the proteins experienced N−H stretch vibrations and the band at 1433 cm−1 is because of methylene scissoring vibrations occurred due to the proteins in the solution. Free amine or cysteine groups embedded within the proteins structure aids the binding process of the proteins to the nanoparticles [17, 23, 25, 27]. The fungus secrets extracellular proteins which are responsible for the reduction of Ag+ to Ag0 and acted as surface capping agent of the synthesised nanoparticle.
Figure 5. FTIR spectrum of AgNPs.
Download figure:
Standard image High-resolution image3.6. TEM and dynamic light scattering analysis
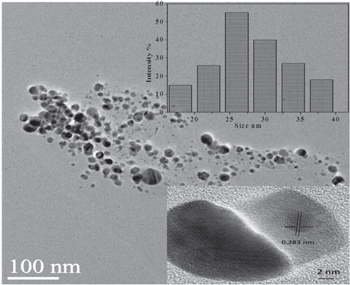
The morphological and dimensional examination of the silver nanoparticles synthesized by Aspergillus wentii NCIM 667 culture filtrate was accomplished utilizing high resolution transmission electron microscopy (figure 6). Prepared Ag nanoparticles retain spherical shape, usual common to microbe intervened synthesis [28] with a typical diameter measuring approximately 25–30 nm. Particle size acquired from TEM image nearly coordinates with that of the dynamic light scattering (DLS) results shown in figure 6 (inset). The size (diameter) of these silver nanoparticles inferred from the size distribution profile differs within 15–40 nm. The asymmetric distribution is especially significant where the majority of the particles stay within 25–30 nm. The gap between the planes is measured to be roughly 0.283 nm that corroborates to the 122 planes of the biosynthesized Ag nanoparticles [17, 23, 27].
Figure 6. HR-TEM image of synthesised AgNP; inset DLS for particle size measurement.
Download figure:
Standard image High-resolution image3.7. Antimicrobial activity assay
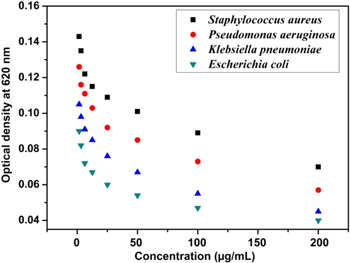
Antibacterial sensitivity studies of the synthesised AgNPs against E. coli, K. pneumoniae, S. aureus, P. aeruginosa shown in figure 7. The MIC values were found to be 1.22 μg ml−1 and 1.82 μg ml−1 for E. coli and K. pneumoniae respectively whereas 2.5 μg ml−1 and 3.1 μg ml−1 were obtained for S aureus and P. aeruginosa respectively. The minimum inhibitory concentration (MIC) values against E. coli and Klebsiella pneumoniae was found to be less as compared to S. aureus and P. aeruginosa as the cell wall of gram negative bacteria is composed of single or double layer of peptidoglycan whereas the cell wall of gram positive bacteria consists of multi-layer peptidoglycan. Therefore the infiltration of Ag nanoparticles into the gram negative bacterial cell was simpler than the gram positive. AgNPs attaches to the surface of the cell membrane initiating rupturing of the membrane thereby accomplishing steady cumulative permeability and seizure of respiratory functions of the cell on other hand the AgNPs might interfere with the components of the microbial electron transport chain leading to the termination of ATP synthesis [28–31].
Figure 7. Minimum inhibitory concentration graph against bacteria for the synthesised AgNPs.
Download figure:
Standard image High-resolution image3.8. Photocatalytic activity of the synthesized AgNPs
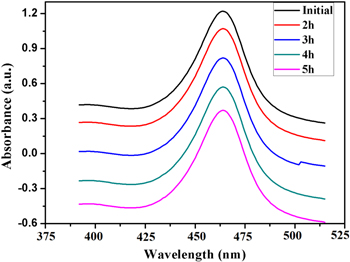
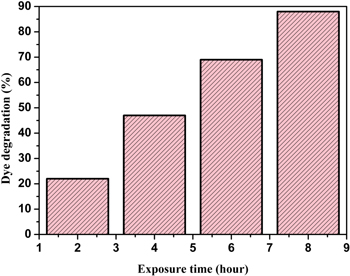
The biosynthesized AgNPs was tested whether they possess the photocatalytic property by virtue of which they would succeed in degrading methyl orange (MO) in presence of solar irradiation. The degradation of the MO dye in presence of AgNPs was established by the steady decrease in absorption peak intensity at 463 nm within 5 h of experimental run as the peak of absorption for the dye is around 460 nm shown in figure 8. On the other hand the control sample exhibited no variation of colour or peak intensity in the time frame of the experiment. From the figure 9, it can be inferred that 88 % degradation of methyl orange was achieved upon treatment with the synthesized AgNPs in presence of sunlight. The percentage of dye degradation and its variation with exposure time is calculated according to following formula
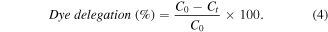
In this formula C0 represents the initial concentration of methyl orange while Ct is the concentration of the methyl orange dye after exposure to solar radiation for t hours. Concentrations of methyl orange were measured considering the absorbance value at 463 nm recorded using UV-Vis spectroscopy. When compared with other irradiation methods, solar irradiation is more proficient in degrading methyl orange in the prevalence of nanosized metal catalysts [16, 17].
Figure 8. Photocatalytic degradation of methylene blue with reaction time.
Download figure:
Standard image High-resolution imageFigure 9. Percentage of dye degradation versus different exposure time.
Download figure:
Standard image High-resolution imageAqueous AgNO3 is temperamental in nature and decays on being heated or by expose to UV light. Therefore Ag0 is the main item in the wake of recognizing oxygen and nitrogen dioxide liable for the degradation process. The colloidal Ag nanoparticles collides with photons from the sunlight, which excited the conduction electrons of metallic Ag mediated by the surface plasmon resonance effect thereby commencing the process of catalytic degradation of the methyl orange dye. Oxygen atoms on the surface of the Ag nanoparticle solubilized the energized surface electrons forming hydroxyl radical and leaving Ag+ particles dragging towards the anionic methyl orange dye. This led to the absorption of the formed hydroxyl radicals on to the surface of the Ag nanoparticles thereby oxidizing methyl orange atoms into its degraded constituents [16–19].
4. Conclusion
This study built up the first ever account of use of culture filtrate of Aspergillus wentii NCIM 667 towards spherical shaped silver nanoparticle synthesis extracellularly. The procedure portrayed for the synthesis of AgNPs is an eco-accommodating technique as it is free from any dissolvable or poisonous chemicals. In addition it is also equally responsive for large-scale production. The size of the formed AgNPs was found within 15–40 nm. The central composite design perceived the most interfacing parameters and the optimum conditions for the nanoparticle biosynthesis process among limited test information set. Maximum UV-Vis spectra at 455 nm relating to the most noteworthy amount of nanoparticle production was obtained setting up an effective quadratic model. A consistent antimicrobial action of the AgNPs against various gram-positive and gram-negative microorganisms was recorded. The photocatalytic study showed that the biosynthesized AgNPs have dynamic photocatalytic property for degrading methyl orange dye within the sight of daylight.
Acknowledgments
The one of the authors (SB) would like to acknowledge the University of Johannesburg, Johannesburg, South Africa for providing a Post-Doctoral Research Fellowship grant.