Abstract
Two main results are presented in this paper. (i) Silver nanoparticles (AgNPs) with uniform size-distribution and controllability in the range of 20–50 nm were synthesized by seeding and growing at ambient conditions. The single-crystal Ag nano-seeds were created by reduction of AgNO3 in presence of citrate surfactant at 70 °C. Then, importantly, the fresh AgCl precursor was used in the presence of polyvinylpyrrolidone to adjust the reaction rate with ascorbic acid to generate Ag for growing on the surface of single-crystal Ag nano-seeds. The AgNPs size could be well-controlled by varying the amount of Ag nano-seeds while keeping the AgCl precursor concentration to be constant. (ii) The large 2D-arrays with homogeneous and dense monolayers of AgNPs were prepared on ITO substrates by hybrid method, in which the key technological point is the surface functionalization of AgNPs using mixed alkanethiols (dodecanethiol:octadecanethiol = 6:1). We have used the fabricated 2D-arrays from the 50 nm AgNPs as a surface enhanced Raman scattering substrate to take the Raman scattering spectra of rhodamine B (RhB), glucose and viral pathogen (H5N1) at very low concentrations of 10−10 M, 10−12 M and 4 ng μl−1, respectively.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 3.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
The assembly of metallic nanoparticles (NPs) into two-dimensional (2D) arrays has been intensively studied owing to their unique optical properties, known as localized surface plasmon resonance (LSPR). LSPR of NPs is generated by the collective oscillations of free electrons when NPs are irradiated by electromagnetic waves. The size, shape, and local dielectric environments, as well as interparticle interactions have a critical effect on the resonance wavelength and bandwidth of LSPR [1–5]. Among various metals, Au and AgNPs have gained a lot of attention due to their resonance wavelengths in the visible spectral range [2, 6]. Compared to other metals, AgNPs arrays exhibit the highest excitation efficiency in the short-wavelength range of the visible region [1, 6, 7], which is often used as surface enhanced Raman scattering (SERS) substrates for sensors to detect trace amounts of molecules [1, 4, 8, 9], and they are also useful for catalyst applications [10, 11].
Many efforts have been devoted to control the size and shape of Ag nanostructures by wet chemical methods, including reduction of silver ions in aqueous or nonaqueous solutions [11–14]. However, the synthesis of AgNPs with uniform and controllable size has been an ongoing challenge. Although the synthesis in organic solvent can produce a good size distribution of AgNPs, these AgNPs with organic ligands on the surface have limited their bio-applicabilities [12]. Alternatively, many studies have focused on the fabrication of Ag NPs in water. The common approach to synthesize the aqueous AgNPs is Lee–Meisel technique by using citrate as a stabilizer and as a reducing agent to reduce AgNO3 at boiling [15]. This method could adjust sizes of AgNPs up to 200 nm but the wide size and shape distributions. Then, Schlucker and Gu groups modified the Lee–Meisel method by adding Ag nano-seeds into reaction system [16, 17]. Ag nano-seeds play a role both as a catalyst for reduction of Ag precursor and as a nucleus for growing larger Ag NPs and thus the relatively good control of uniform quasi-spherical Ag NPs in range of 20–30 nm. However, the use of reactive Ag precursor and carrying out the reaction at high temperature for long reaction time usually produces AgNPs with broad size distribution and different shapes because it is very hard to control the NPs growth. Therefore, the use of the lower reactive Ag precursor instead of AgNO3 solution is considered to be a good strategy to produce the large and monodisperse of quasi-spherical AgNPs. Because AgCl suspension is a nearly water-insoluble compound giving rise to the very low concentration of free Ag+ in water that favors the controllability of the growth rate of AgNPs [17].
The major AgNPs-based SERS substrates have been fabricated by top-down methods including various lithograph patterning techniques that allow high spatial resolution and accuracy, reproducibility and periodic arrangement of plasmonic nanostructures [18, 19]. However, these methods are impractical for the large substrates due to their low-production and high cost. While bottom-up approaches based on chemical methods would allow inexpensive and efficient production of 2D arrays with large area. The excellently ordered 2D-arrays from AgNPs were produced by bottom-up approach based on external field induced assembly [5]. However, these AgNPs are only physisorbed on substrates thus lack the mechanical strength required for device materials. Another bottom-up approach is the chemical immobilization by covalent bonding between AgNPs and substrates [3, 20]. Although this AgNP possess high mechanical durability on substrates, but it is difficult to have the high density due to the repulsion force between AgNPs during the immobilization reaction. Recently, we have successfully developed a hybrid method that combines three elemental technologies: (i) chemical modification of both surfaces of metallic NPs and conductive substrates with an alkanethiol and a thiol-terminating agent, respectively; (ii) electrophoresis; and (iii) solvent evaporation, to produce the large-area and close-packed 2D-arrays of AuNPs and Au/Ag core/shell NPs [2, 21, 22]. By using the mixture of the short- and long-chain alkanethiols (such as dodecanethiol:octadecanethiol = 6:1) self-assembled monolayer on surface of AuNPs, we fabricated AuNPs 2D-arrays with high coverage (>80%) and long-term stability [2]. As far as we know, there has not been any report on the production of AgNPs 2D-arrays by using the hybrid method.
In this paper we report the two main results on (i) the synthesis of monodisperse AgNPs and (ii) the arrangement of AgNPs to make 2D-arrays. Based on the technological analyzes of the syntheses available in the literature, we take the appropriate advantages to develop an efficient synthesis method, namely the seed-mediated growth of AgNPs using the lower reactive AgCl precursor in the presence of polyvinylpyrrolidone (PVP). This method enables to control the reaction rate between AgCl and ascorbic acid that produces fresh Ag for continuing the growth of Ag nano-seeds to become bigger AgNPs of the designed sizes. AgNPs with uniform size-distribution in the range of 20–50 nm were synthesized at ambient conditions. After obtaining good AgNPs, we fabricated the large 2D-arrays with homogeneous and dense monolayer of AgNPs on indium tin oxide (ITO) substrates by hybrid method, in which the key technological point is the surface functionalization of Ag NPs using mixed alkanethiols (dodecanethiol:octadecanethiol = 6:1). Finally, to test the fabricated 2D-arrays of Ag NPs as SERS substrates, we could take the Raman scattering spectra of RhB, glucose and H5N1 virus at very low concentrations of 10−10 M, 10−12 M and 4 ng μl−1, respectively.
2. Experimental
2.1. Chemicals
1-octadecanethiol, 1-dodecanethiol, 3-mercaptopropyl trimethoxysilane (MPTMS), 1,6-hexanedithiol, silver nitrate, trisodium citrate, ascorbic acid, sodium chloride, polyvinylpyrrolidone (PVP40) were purchased from Sigma-Aldrich. Sodium borohydride was purchased from Merck. All chemicals were guaranteed reagent grade and were used as received. The cleanly level of flask, beaker and stirring bars were affected to size distribution and shape monodispersity. Therefore, all the glassware and magnetic stirring bars were soaked in a fresh aqua regia solution for about an hour, followed by a rinse with de-ionised water (DI), and then washed with ethanol before drying in oven.
2.2. Synthesis of Ag nano-seeds
Ag nano-seeds in colloidal solutions were prepared following the procedure described in [17]. Briefly, 50 ml of 8 mM trisodium citrate solution was placed in a flask and heated to 70 °C for 10 min. Next, 0.85 ml of 60 mM AgNO3 aqueous solution was injected into the flask containing citrate, followed by the quick addition of 1 ml of 26 mM NaBH4 solution, which was freshly prepared, for reduction of AgNO3 to produce Ag nano-seeds. The reaction solution was kept at 70 °C under vigorous stirring for 1 h and cooled down to room temperature. This single-crystalline Ag nano-seeds colloid was stored at 4 °C for further obtaining larger Ag NPs (of 20, 30, 40 and 50 nm) by the seed-mediated growth.
2.3. Size-controllable synthesis of AgNPs
AgNPs of various sizes in colloidal solutions were synthesized by the seed-mediated growth method, in which single-crystalline Ag nano-seeds and the fresh AgCl suspension in the presence of PVP played an important role. Particle sizes of AgNPs could be controlled by varying the amount of Ag nano-seeds while keeping the AgCl precursor concentration to be constant. Briefly, 10 ml of ascorbic acid (100 mM) was dropped with the rate of 0.6 ml min−1 into a beaker containing the mixture of 2.5 ml of fresh PVP-capped AgCl (25 mM) solution, 30 ml of DI water and the amount of 6.27 ml, 1.73 ml, 0.72 ml and 0.37 ml for the 20 nm, 30 nm, 40 nm and 50 nm expected final diameter, respectively of starting colloidal solution (∼5 nm seed) in a strong stirring for 1 h. The fresh PVP-capped AgCl solution was prepared by adding 85 mg of AgNO3 into 20 ml aqueous solution containing 85 mg of PVP40, followed by an injection of 200 μl of NaCl (5 M) under rapid stirring for 15 min.
2.4. Functionalization of AgNPs
AgNPs colloidal solutions (10 ml each) were mixed with 10 ml of mixed alkanethiol of dodecanethiol and octadecanethiol (100 mM) with the molar ratio of 6:1 in acetone and stirred at room-temperature overnight. Then mixed alkanethiol-capped AgNPs were extracted by using mixture of acetone and n-hexane, and then purified by sequential centrifuge and redispersion into the mixed acetone–hexane solvent for three times (with the acetone:hexane volume ratio of 10:1, 1:1, 1:9, respectively). After the final rinse cycle, the supernatant was discarded and the thiol-capped AgNPs were dried under N2 environment, producing the stable black powder, which could be redispersed in organic solvent and ready for fabricating 2D arrays.
2.5. Functionalization of ITO substrate
Cleaned quartz substrates (10 mm × 10 mm × 0.625 mm) were coated with ITO (10 nm) by sputtering. These substrates were immersed in a 20 ml of 1 vol% of 3-mercaptopropyl trimethoxysilane (MPTMS) in toluene for 36 h, rinsed with methanol for three times and dried in a nitrogen-gas environment. Subsequently, these substrates were immersed in a 20 ml of 1 vol% of 1,6-hexanedithiol in ethanol for 12 h, rinsed with isopropanol for three times and dried in a nitrogen-gas environment.
2.6. Fabrication of 2D-arrays of AgNPs by hybrid method
The thiol-terminated ITO substrate as cathode- and plastic formed carbon (10 mm × 10 mm × 1.0 mm) as anode-electrodes were placed 1.0 mm apart from each other and immersed in a 2 ml of alkanethiol-capped AgNPs solutions in the hexane–acetone solvent (9:1 in the vol ratio), followed by an application of 1.0 V as bias voltage for the electrochemical deposition. After the solvent evaporation was completed, the samples were annealed at 50 °C for 12 h to immobilize the AgNPs on the functionalized ITO substrates by substitution of thiol. Finally, the samples were sonicated in n-hexane for 30 s to remove AgNPs physisorbed on the chemisorbed monolayer. Ag with different sizes of 20, 30 , 40 and 50 nm were used to fabricate the 2D-arrays.
2.7. Characterization
Morphology of the synthesized AgNPs and 2D-arrays made from them were visualized by the SEM images (using a Hitachi S-4800 FE-SEM). Extinction of the 2D-arrays made from AgNPs was estimated via recording the absorption spectra using an UV–vis-NIR double-beam spectrometer (Varian Cary 5000) equipped with a liquid cell holder module for colloidal samples and a transmission module for solid substrates. To test the obtained 2D-arrays of AgNPs as SERS substrates, the Raman scattering spectra of RhB, glucose and H5N1 virus at very low concentrations of 10−10 M, 10−12 M and 4 ng μl−1, respectively, on a typical 50 nm AgNPs array were taken using a QE6500 spectrometer (Ocean Optics) equipped with a Nd:YAG laser (532 nm, 5 mW power at the sample) as the excitation source, a Hamamatsu (S7031-1006 back-thinned, TE cooled to −10 °C to reduce dark noise) CCD array as detector. H5N1 virus samples of different concentrations were prepared following the safe biotechnology procedure.
3. Results and discussion
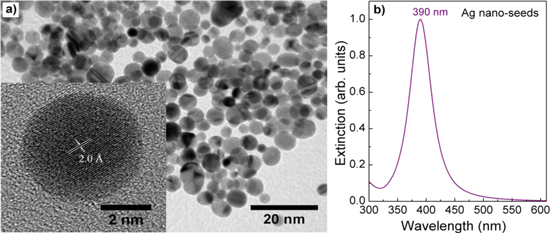
Figure 1 shows the TEM image of Ag nano-seeds and the extinction spectrum taken from them in colloidal form. The TEM image shows Ag nano-seeds in rather spherical uniform with the size of 5 nm. In a typical high-resolution TEM image (see the inset in figure 1(a)), one can see a good crystalline Ag nano-seed with the lattice fringe spacing of 2.0 Å, which is consistent with the spacing between the (200) planes of face-centered cubic Ag [23]. The single-crystalline seed is one of the key factors for controlling the spherical AgNPs because it favors the evolvement of isotropic structures. If the nano-seeds are just clusters or contain the twin planes that would favor the formation of Ag nanorods during the growth [24, 25]. The sharp extinction at 390 nm (figure 1(b)) indicates clearly the characteristic surface plasmon resonance of AgNPs and the narrow-sized distribution [17].
Figure 1. (a) TEM image (inset: HR-TEM image showing the crystalline structure) of Ag nano-seeds and (b) corresponding extinction spectrum.
Download figure:
Standard image High-resolution imageThe larger-sized AgNPs were synthesized by changing the amount of Ag nano-seeds into a certain quantity of fresh AgCl/PVP and ascorbic acid precursors. As shown in figure 2, the spherical and uniform AgNPs with the size increasing from 20 to 30, 40, and 50 nm were obtained by adding Ag nano-seeds in 6.27 , 1.73 , 0.72 and 0.37 ml to the solution containing 2.5 ml of fresh PVP-capping AgCl suspension, followed by the drop of 10 ml of ascorbic acid. With decreasing the number of Ag seeds in reaction solution while keeping the quantity of AgCl precursor fixed, the size of AgNPs is increased. This means that the amount of AgCl precursor mostly provide Ag source for growing on the surface of Ag nano-seeds to make AgNPs bigger. Because the reduction rate of ascorbic acid with fresh AgCl/PVP suspension occurs gradually that favor the growth of Ag nano-seeds with freshly generated Ag atoms. Moreover, PVP molecules adsorbed on the surface of Ag nano-crystals make the crystallites isotropic [14]. Consequently, the size and shape of AgNPs could be efficiently adjusted by the growth of Ag crystallites using a mild reducing agent of ascorbic acid to reduce the fresh AgCl/PVP water-insoluble precursor at room temperature. For testing different Ag precursors, the AgNO3 solution was used instead of AgCl/PVP while other parameters were kept the same. However, the black precipitates were immediately appeared during the addition of ascorbic acid into the mixed solution of Ag nano-seeds and AgNO3 precursor. This indicates that the reaction of ascorbic acid and AgNO3 occurs too fast and the generated AgNPs were aggregated together.
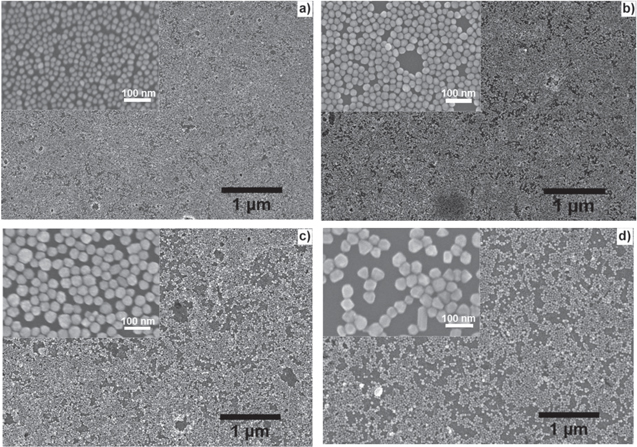
Figure 2. SEM images of AgNPs 2D arrays of thiol-capped particles prepared with different sizes: (a) 20 nm, (b) 30 nm, (c) 40 nm and (d) 50 nm. Insets: the same images at higher magnification.
Download figure:
Standard image High-resolution imageIn order to assemble AgNPs to make 2D-arrays, we have selected the mixture of dodecanethiol and octadecanethiol for functionalization of AgNPs and MPTMS for that of the ITO-coated quartz substrates. The functionalization of both AgNPs and substrates could make the self-assembled monolayer of AgNPs with highest coverage on the substrates. This is based on our previous study for AuNPs that showed the mixed alkanethiolate of dodecanethiol and octadecanethiol with the molar ratio of 6:1 could give the highest coverage of alkanethiol-capped Au colloidal films [2]. In the present study, AgNPs arrays were fabricated by the self-assembly of functionalized AgNPs on the ITO-coated quartz substrates, similar to the AuNPs arrays case. Figure 2 shows the SEM images of 2D-arrays made from the mixed alkanethiol-capped AgNPs with different sizes. One can see the close-packed with large-area and regularly ordered 2D arrays of AgNPs. The substrate is almost covered with smaller AgNPs (figures 2(a) and (b)), while relatively large uncovered domains are seen with the larger sizes AgNPs (figures 2(c) and (d)). For larger AgNPs, the van der Waals attraction force among AgNPs increases rapidly and the steric repulsive force is not sufficient for countering long-range van der Waals attraction force that lead to the low dispersion stability of AgNPs. For small AgNPs, the preferential adsorption of the shorter-chain alkanethiol is sufficient and is maintained by a high curvature of particles. As a result, better dispersion stability of small AgNPs is achieved. The good dispersion stability of AgNPs in hydrocarbon solutions contributes to the high surface coverage of 2D arrays [2]. We have found that the coverage of AgNPs was less dense than that of AuNPs in the same treatment conditions. There are two features that must be pointed out for the 2D-arrays made from the mixed alkanethiol-capped AgNPs: (i) the surface coverage of AgNPs is not full, and (ii) the periodic arrangement of AgNPs is not possible. We did successfully the 80% coverage of AgNPs on substrates that is very dense compared to the published data [20–22]. To have practically the periodic 2D arrays of Ag or Au NPs one need to fabricate them by using physical method like nano-lithograph patterning technique that enables the high spatial resolution, accuracy, reproducibility and periodic arrangement of the nanostructures. These physical methods are almost impractical to produce large SERS substrates because they require expensive equipments and the production is normally very low (as an example, we have ever fabricated a periodic 2D array of 150 nm gold on Si wafer by nano-lithography; it took 5 h to obtain a small-area of 20 × 20 μm2). In the present case, 2D arrays of AgNPs have been made by chemical method, which allows inexpensive and efficient fabrications of 2D arrays with large area (square centimeter) and high density that formed 'hot-spots' between AgNPs to induce the great enhancement of the electromagnetic field, consequently making SERS activity very efficient.
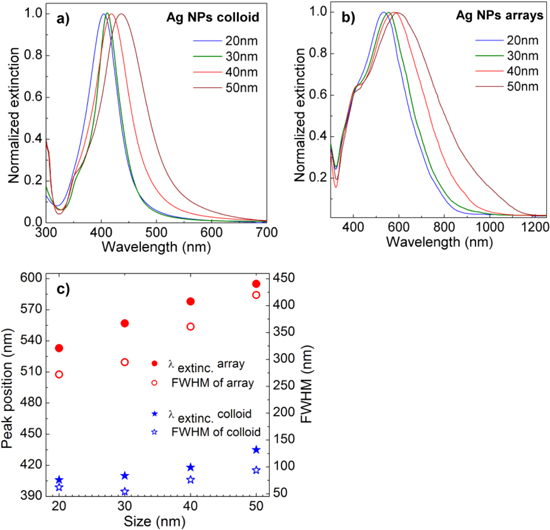
Figure 3 shows the UV–vis-NIR extinction spectra, peak position and bandwidth taken from AgNPs in different sizes in the colloidal and 2D-arrayed forms. The sharp plasmon resonance peak of colloidal AgNPs (figure 3(a)) confirms the narrow size distribution of the synthesized AgNPs. It can be also seen the tendency of broader size distribution with the larger AgNPs. This is because the relatively easy oxidation of AgNPs often occurs during the reduction process of Ag ions resulting in a broad size distribution of large AgNPs. Consequently, the synthesis of the large uniform-sized AgNPs is more difficult compared to the corresponding AuNPs case. The obtained UV–vis-NIR extinctions are consistent with the observation from the SEM images presented in figure 2. As shown in figures 3(a) and (b) the LSPR peaks of Ag NPs in colloidal form are linearly red-shifted in the range of 405–435 nm, while those of AgNPs in the 2D-arrays show the linear red-shift from 533 nm to 595 nm, as their sizes increased from 20 nm to 50 nm, respectively. The much larger red-shift of the plasmon resonance peak of AgNPs in 2D-arrays compared to that of AgNPs in colloidal form is resulting from the strong interaction between the close AgNPs in 2D-arrays. Along with the red-shift of the plasmon resonance peak, the bandwidth of bigger particles is rapidly broadened (figure 3(c)). This could be attributed to the broader size distribution in larger AgNPs particles [20]. The plasmon resonance range of 533–595 nm from the fabricated AgNPs 2D-arrays is very suitable for the laser excitation sources to take the Raman scattering spectra with great enhancement. In other words, they can serve well for SERS applications.
Figure 3. UV–vis-NIR extinction spectra of AgNPs in (a) colloidal form, (b) 2D-arrays, and (c) the peak positions (λextinc) and bandwidths (FWHM) as a function of their sizes.
Download figure:
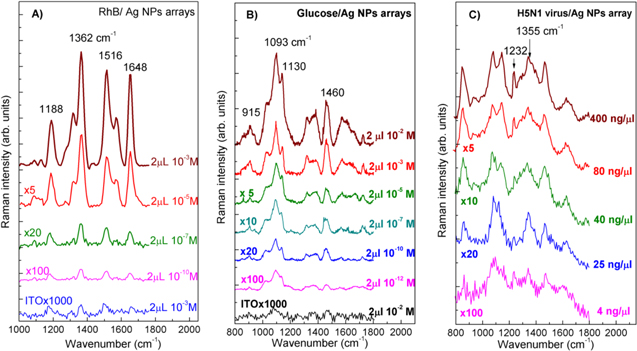
Standard image High-resolution imageTo estimate the SERS responses of the fabricated AgNPs arrays, RhB was used as a probe molecule to take the Raman spectra. One of the main reasons for using RhB dye to measure its Raman scattering spectra is that RhB dye is a very strong photoluminescence material, consequently it is almost impossible to record the Raman signal by conventional techniques. In other words, RhB (with luminescence quantum yield of 65%) is really a tough target for taking Raman scattering signal and is an excellent reporter for the SERS activity. Only with the great enhancement by surface plasmon resonance, Raman scattering signal becomes strong enough compared to the luminescence to enable measuring Raman scattering spectra of RhB dye. Figure 4(A) shows the Raman scattering spectra of RhB at various concentrations dropped onto the typical 50 nm AgNPs array and onto the bare ITO substrate for comparison, while keeping the same parameters such as the volume of probe molecules, the excitation laser wavelength and power. The characteristic Raman peaks of RhB molecule with aromatic C–C stretching vibrations at 1648, 1570 and 1362 cm−1, and C–H bending at 1315 cm−1, and C–C bridge-band stretching at 1188 and 1510 cm−1 were observed; these are in good agreement with the results reported in the literature [26, 27]. With decreasing the RhB concentration from 10−3 M to 10−10 M, the Raman scattering intensity decreased. We could take the Raman scattering spectrum of RhB dye with the concentration as low as 10−10 M, with the 50 nm AgNPs array as a SERS substrate. The Raman scattering enhancement is usually explained as inducing from the 'hot spots' located at the nanogaps between AgNPs that come from surface plasmon resonances appearing once the metallic particles are excited by an appropriate laser beam [1, 4]. Therefore, the enhancement of electromagnetic fields at the hot spots results in the enhancement of Raman scattering of molecules located on the surface of AgNPs. For ITO substrate without any AgNPs, the Raman scattering signals from RhB molecules were not observed for even the high concentration of 10−3 M. To approach further with possible applications of the fabricated SERS substrates, we have used them to take the Raman scattering spectra of glucose and a viral pathogen, H5N1 virus in the present case. Figures 4(B) and (C) show the Raman scattering spectra of glucose and H5N1 virus, respectively, at various concentrations and that of the bare ITO substrate for comparison. It is seen that at very low concentrations of 10−12 M and of 4 ng μl−1, respectively, glucose and H5N1 virus could be observed directly via their Raman scattering spectra with the specific lines at 915 , 1093 and 1130 cm−1 (for glucose), 1232 and 1355 cm−1 (for H5N1 virus), in consistence with published data [28, 29]. Note that with viral pathogen one can take mostly the Raman scattering spectra from the proper protein on their surface.
Figure 4. Raman scattering spectra of (A) RhB, (B) glucose and (C) H5N1 virus at different concentrations loading on a SERS substrate fabricated from the 50 nm AgNPs. The concentrations and multiplication factors are indicated at the spectra.
Download figure:
Standard image High-resolution image4. Conclusion
We have demonstrated the use of seed-mediated growth as an effective approach to synthesize the monodisperse spherical AgNPs with facile controllable sizes in the range of 20–50 nm. We have found that the important point to synthesis AgNPs with uniform size-distribution is the use of fresh AgCl colloid in the present of polyvinylpyrrolidone to adjust the reaction rate of AgCl and ascorbic acid to generate Ag for growing on the surface of Ag nano-seeds. The AgNPs sizes could be well-controlled by varying the amount of Ag nano-seeds while keeping the AgCl precursor concentration to be constant. We have succeeded in arranging AgNPs to fabricate the large, homogeneous and dense 2D-arrays by using hybrid method, in which the key technological point is the surface functionalization of AgNPs from mixed alkanethiols (dodecanethiol:octadecanethiol = 6:1). We have used the fabricated 2D-arrays of 50 nm AgNPs as SERS substrates to take the Raman scattering spectra of RhB, glucose and H5N1 virus at very low concentrations of 10−10 M, 10−12 M and 4 ng μl−1, respectively. The results show the SERS substrates chemically made from AgNPs are very promising for detection of trace biomolecules.
Acknowledgments
This work was supported by the East Asia Science and Innovation Area Joint Research Program (e-ASIA JRP), Ministry of Science and Technology (MOST Vietnam). We thank the National Key Laboratory for Electronic Materials and Devices (VAST/IMS) for the use of facilities.