Abstract
The combination of one-dimensional (1D) carbon nanotubes (CNTs) and two-dimensional (2D) graphene materials to generate three-dimensional (3D) carbon nanotube–graphene hybrid thin films (CNGHTFs) has attracted great attention owing to their intriguing properties via the synergistic effects of these two materials on their electrical, optical, and electrochemical properties in comparison with their individual components. This review aims to provide a brief introduction of recent trends in preparation methodologies and some outstanding applications of CNGHTFs. It contains two main scientific subjects. The first of these is the research on preparation techniques of CNGHTFs, including reduction agent-assisted mechanical blending of reduced graphene oxide (rGO) and CNTs, hybridization methods for layer-by-layer (LBL) assembly of CNTs and rGO sheets, multi-step methods using combinations of a solution and chemical vapor deposition (CVD) processing, one-step growth of CNGHTFs by the CVD method, and modified CVD methods via thermal deposition of carbon source on catalyst surfaces. The advantages and disadvantages of the preparation methods of CNGHTFs are presented and discussed in detail. The second scientific subject of the review is the research on some outstanding applications of CNGHTFs in various research fields, including transparent conductors, electron field emitters, field-effect transistors, biosensors and supercapacitors. In most cases, the CNGHTFs showed superior performances than those of the pristine GO/graphene or CNT materials. Therefore, the CNGHTFs exhibit as high-potential materials for various practical applications. Opportunites and challenges in the fields are also presented.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 3.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Owing to its unique optical, electrical and mechanical properties, graphene has emerged as a new class of promisingly attractive materials for applications such as transparent electrodes [1–5], optoelectronics [6, 7], field-effect transistors [8–10], energy storage materials [11–13], biosensors [14–17], and composites [18, 19]. In recent years, combinations of one-dimensional (1D) carbon nanotubes (CNTs) and two-dimensional (2D) graphene sheets to form flexible three-dimensional (3D) CNT–graphene hybrid thin fims (CNGHTFs) have attracted great attention owing to their intriguing properties. The synergistic effects of these two carbon materials improve the electrical, optical, electrochemical, and mechanical properties of CNGHTFs [20–30]. CNGHTFs have an interconnected network of carbon structures with well-defined nanopores, leading to a synergistic effect in enhanced conductivity. Thus, they are suitable for a variety of applications, such as transparent conductors, and electron field emitters [31–34], field-effect transistors [35, 36], sensors [37–41], and energy storage [42, 43].
To date, the preparation of CNTs is becoming mature while graphene fabrication is still being developed. In general, graphene oxide (GO) is preferably selected for hybridization with CNTs because of its potential mass production at low cost. Solution processing, however, involves the use of reduced graphene oxide (rGO) and oxidized or functionalized CNTs, which requires complicated chemical routes with highly toxic agents and solvents for synthesis [20]. To overcome this issue, some research groups have prepared CNGHTFs by chemical vapor deposition (CVD) [23, 25, 44–46]. Another method to prepare CNGHTFs is the deposition of GO platelets from aqueous colloidal suspensions, followed by metal catalyst particles decorated with the GO platelets on various spin-coated substrates during the processings using CVD [35], or modified CVD via thermal decomposition of carbon source on direct-heated catalyst surfaces [27]. Although the aforementioned methods represent great advantages for CNGHTFs production, a facile and efficient approach for direct bonding between CNTs and graphene is still a big challenge.
In this review we present an overview of recent researches on CNGHTFs, with a focus on the preparation methods. In the following parts, we will first review the preparation methods of graphene and CNTs, and then describe some popular methods for fabricating CNGHTFs. The advantages and disadvantages of these preparation methods are presented and discussed in detail. Next, some applications of CNGHTFs in various research fields, including transparent conductors, electron field emitters, field-effect transistors, biosensors and supercapacitors are briefly presented. Finally, we discuss the challenges and opportunities for future research toward the controlled growth and performance applications of CNGHTFs.
2. Preparation techniques of CNGHTFs
Several methods have been developed for preparing graphene sheets since it was firstly isolated by Novoselov and Geim using Scotch tape in 2004 [47], such as electrochemical exfoliation [48–50], arc discharging [51, 52], mechanical milling [53, 54], expanded graphite-based exfoliation [55–59] and chemical reduction of exfoliated graphite oxide [60–63]. CNTs are also synthesized by methods commonly employed in graphene production. The detailed preparation methods, properties, and applications of CNTs can be found in the previous literature [64]. Various techniques have been employed for fabricating CNGHTFs, including layer-by-layer stacking [22] and hybridization of CNT networks and graphene [29]. Recently, researchers have introduced some other mehthods of partial unzipping/splitting of CNTs via metal-assisted thermal etching [44], potassium vapor intercalation followed by solvent quenching [45], and deposition of GO platelets from aqueous colloidal suspensions, followed by metal catalyst particles decorated with GO platelets on various spin-coated substrates during CVD processing [35]. This section briefly describes some recently developed methods for CNGHTFs synthesis.
2.1. Reduction agent-assisted mechanical blending of rGO and CNTs
Reduction agent-assisted mechanical blending of rGO and CNTs is the simplest method for taking advantage of the merits of each carbon nanomaterial via combining GO and CNTs, followed by reduced processing from GO to rGO [26]. In this method, ultrasonication or rotation-based mixing is often used to disperse CNTs in an aqueous solution of rGO. Graphene nanosheets, which have a larger area per unit mass than that of CNTs, are employed as the conductive pathways. Song et al [20] produced conductive hybrid films, consisting of CNTs and GO nanoplatelets. The square resistance of the hybrid films relies on the percentage of the percolated CNT network. This technology is facile, inexpensive, scalable, and has potential for application in conductive materials for electronic devices. However, good dispersion of CNTs in aqueous solution is not always achieved, due to the hydrophobic characteristics and the strong van der Waals tube attraction [65]. Therefore, it remains to be a challenging endeavor to realize hybrid structures with uniform blends of CNTs with rGO and well-defined compositions and structures at the nanoscale level. A promising approach to overcome the problem is assembly of CNTs with graphene sheets using the electrostatic interactions between positively charged multi-walled carbon nanotubes (MWCNTs) and negatively charged exfoliated nanosheets of rGO.
2.2. Layer by layer assembly of GO/rGO and CNTs
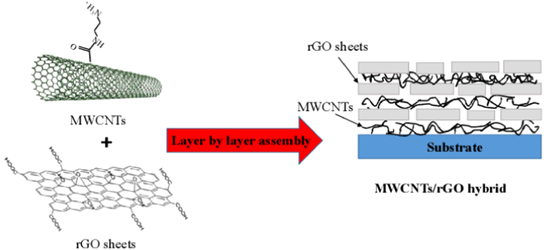
Early hybridization methods for layer-by-layer (LBL) assembly of CNTs and rGO sheets are the wet blending of CNTs and rGO sheets and solid-phase layer stacking of CNT networks on a graphene film [32]. There are several primary advantages of LBL hybridization. First, it offers an uniform dispersion of CNTs into the composite, which is enabled by direct adsorption of CNTs from a solution to a solid state without phase segregation. Second, hybrid composites that are enabled by accurately controlled multi-component nano-layers have tunable multifunctional properties. Third, the methods are simple, robust, and offer versatile processibility for producing large-scale CNGHTFs by controlling the experimental parameters (especially for producing nano-thin composite coatings) [66]. The early pioneering works on LBL CNTs are the successful conquests over dispersion challenges of CNTs in polymer composites [67–70]. Figure 1 shows a schematic of the hybrid multi-layers formed by employing the electrostatic interactions between positively charged MWCNTs and negatively charged exfoliated nanosheets of rGO. This method allows good control of the optical and electrical properties for producing transparent and conducting thin films with a sheet resistance of 8 kΩ sq−1 and a transparency of 81% at 550 nm, resulting from assembly parameters such as concentration, pH, and spin-assembly conditions [32]. Hybrid carbon films were formed by sequential self-assembly of the functionalized 2D graphene sheets and 1D CNTs via electrostatic interactions onto various substrates [69]. The films were successfully interconnected carbon structures with well-defined nanoscale pores, and thus they exhibited a rectangular cyclic voltammogram (CV) even at a high scan rate of 1 V s−1 and the average specific capacitance of 120 F g−1. These results is promising for the uses of the hybrid carbon films as the electrodes in high-performance supercapacitors [69].
Figure 1. Schematic illustration of CNT and rGO hybrid fabrication via the electrostatic interactions between positively charged CNTs and negatively charged exfoliated nanosheets of rGO.
Download figure:
Standard image High-resolution imageThe LBL process of CNGHTF fabrication involves the assembly of oppositely charged interaction between GO/(rGO) and CNTs or between mixed graphene sheets and CNTs, in dispersions with or without surface-funtionalization of CNTs in various polymers through different types of interactions [71]. The function of single-walled carbon nanotubes (SWCNTs)/MWCNTs not only provides the electronic conductivity, but also affords mechanical flexibility for the hybrid film, while allowing for electronic contact amongst graphene nanosheets by bridging their gaps [67]. The sheet resistance and optical transmittance of the as-prepared hybrid film reach 400 Ω sq−1 and 84% respectively. These results suggest a great potential for fabricating ultra-high-performance transparent conductors. However, this approach usually produces 2D structures without well-controled morphologies, and thus it limits the practical applications. Various attempts have been developed to construct 3D hybrid nano-architectures to engineer the number layers of graphene for satisfying the requirements of a specific application such as transparent conductive electrodes [72, 73]. Clearly, all the mentioned methods involve difficultly in constructing covalent C–C bondings between graphene layers and CNTs in the hybrids, which can significantly limit performance in practical applications. Therefore, recent attention has been focused on efficient methods for fabricating CNGHTs that provide covalent C–C bondings between graphene layers. One of the most direct and simple ideas is to grow CNTs on GO or rGO surfaces by coating catalyst nanoparticles in CVD processing.
2.3. Multi-step methods using combinations of solution and CVD processing
CVD is the most promising method that directly grows CNTs, graphene, or both CNTs and graphene on a substrate surface. Thus, it will greatly facilitate the fabrication of all carbon-based nanoelectronics. The CVD process is a catalytic conversion of a gaseous precursor at high temperatures into a solid material at the surface of catalyst particles [28]. Figure 2 shows a schematic of thermal CVD process for growing SWNTs on rGO films. In this method, large-area graphene films are usually produced by deposition of GO platelets in aqueous colloidal suspensions, followed by decorating the platelets with metal catalyst nanoparticles. Then, CNT growth is promoted by segregating the nanoparticles on various spin-coated substrates during the CVD process for CNGHTF fabrication [33]. The major advantages of CVD-based methods are efficiency, directness and high yield and the ability to produce all carbon-based devices. Therefore, fabrication of CNGHTFs has mostly employed these methods. For example, CNT network embroidered graphene films with high conductivity have been sucessfully prepared using hybridization of CNT networks and graphene. The formation mechanism of the CNT network embroidered graphene films can be summarized as follows: first, in the nucleation stage, defects on the CNT walls are catalytically etched by Cu at high temperatures to result in a high density of carbon adatoms near the CNTs. Consequently, the initiating graphene nuclei are formed. Second, during the enlargement of graphene domains, large CNT bundles play the role of diffusion barriers of carbon adatoms to regulate effectively the shapes of the growing graphene domains. Finally, the CNTs and graphene coalesce into a continuous film. The presence of CNTs on or in graphene films results in a significantly decreased sheet resistance without seriously reducing the transparency [29]. Similarly, SWCNTs and graphene were combined successfully by adjusting the spin-coating speed for SWCNTs suspension on UV-treated Cu foil. Subsequently, graphene was synthesized on the SWCNTs/Cu foil using thermal CVD (TCVD) [74]. The successful combination of SWCNTs and graphene was applied for fabricating the semiconducting SWCNTs-graphene hybrid field-effect transistor (FET) with high on/off ratios and electrical conductivities suitable for high-performance flexible devices [74].
Figure 2. Schematic of CVD process for growing SWCNTs on rGO films.
Download figure:
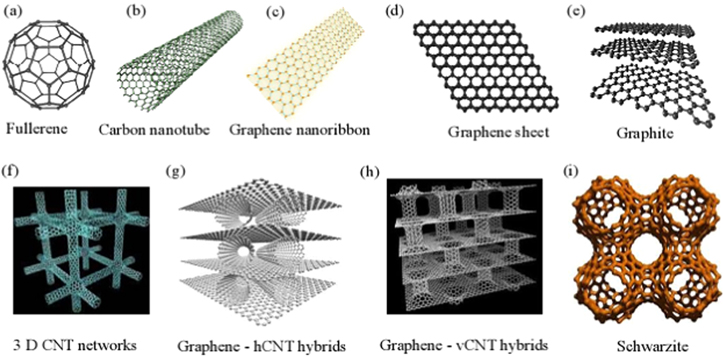
Standard image High-resolution imageClearly, tremendous efforts have been devoted in recent years to integrate individual CNTs into dimensionality of sp2-hybridized carbon nanostructures to achieve different configurations of graphene–CNT hybrid nanostructures. Indeed, figure 3 shows the different configurations of carbon nanostructures: fullerene (0D) (figure 3(a)), carbon nanotube (1D) (figure 3(b)), graphene nanoribbon (1D) (figure 3(c)), graphene sheet (2D) (figure 3(d)), graphite (3D) (figure 3(e)), 3D CNT networks (figure 3(f)), hybrid of graphene with horizontal CNT (hCNT) (3D) (figure 3(g)), hybrid of graphene with vertical CNT (vCNT) (3D) (figure 3(h)), and graphene triple periodical minimal surfaces (or schwarzites 3D graphene) (figure 3(i)). Hybrid nanostructures of CNT and graphene can be classified into two main categories, namely CNT-rich hybrids and graphene-rich hybrids [24]. Figure 4 shows schematics of different configurations of graphene–CNT hybrid nanostructures: CNT-rich hybrids (graphene sheets un-zipped from the outer walls of CNTs (figure 4(a)) and graphene sheets derived from the inner walls of CNTs (figure 4(b)) and graphene-rich hybrids (CNTs stood vertically on graphene sheets to form pillared arrays (figure 4(c)) and CNTs spread horizontally on graphene sheets (figure 4(d)). More recently, Tour et al [23] demonstrated that graphene and SWNTs are overlapped via π–π stacking rather than covalent welding, resulting in good planar CNTs/graphene hybrids (termed as rebar graphene). The CNTs act as reinforcing bar (rebar), toughening graphene through both π–π stacking domains and covalent bonding where the CNTs partially unzip and form a seamless 2D conjoined hybrid [75]. This type of hybrid possesses a transmittance of ∼95.8% at 550 nm and a sheet resistance of ∼600 Ω sq−1, indicating better performance than those of stacked CVD bilayer graphene or CNT films at the same transimittance [75].
Figure 3. Schematic presentation of dimensionality of sp2-hybridized carbon nanostructures. (a) Fullerene (0D); (b) carbon nanotube (CNT) (1D); (c) graphene nanoribbon (1D); (d) graphene sheet (2D); (e) graphite (3D); (f) 3D CNT networks (3D); (g) hybrid of graphene with horizontal CNT (hCNT) (3D); (h) hybrid of graphene with vertical CNT (vCNT) (3D); and (i) graphene triple periodical minimal surfaces (or schwarzites 3D graphene).
Download figure:
Standard image High-resolution imageFigure 4. Schematic presentation of different configurations of graphene carbon nanotube hybrid nanostructures: (a) graphene sheets un-zipped from the outer walls of CNTs, (b) graphene sheets derived from the inner walls of CNTs, (c) CNTs stood vertically on graphene sheets to form pillared arrays, (d) CNTs spread horizontally on graphene sheets.
Download figure:
Standard image High-resolution imageFor simultaneous formation of CNT networks on graphene films, an alternative method is to rapidly heat and cool in CVD processing using copper foil and acetylene as catalyst and carbon feedstock gas (figure 5). The as-prepared CNT–graphene hybrid film exhibits a decrease in sheet resistance down to 420 Ω sq−1 and a transmittance of light of 72.9%. In this case, graphene acts as a barrier layer and plays an important role in preventing the diffusion of iron into the copper substrate. CNTs can be attributed to the formation of heterogeneously oriented iron nanoparticles, which are induced by rough surface of graphene/copper sample. The densities of thin CNTs networks are controlled by varying the thickness of iron catalysts, integrated on the graphene surfaces through the same CVD process [44]. The as-grown graphene and CNT–graphene hybrid films were transferred onto polymer substrates for flexible transparent conductor applications. Additionally, CNT–graphene hybrid films have been demonstrated to be effective field electron emitters because of the low contact resistance via π–π interactions between the two types of graphitic material. These findings may be used in applications of flexible electronics where controlled opto-electronic and field emission properties are desired [33]. Recently, Chuc et al [44] also successfully synthesized CNGHTFs on polycrystalline Cu substrates by an atmospheric pressure CVD method. Ferric salt FeCl3 solution was used as a precursor for CNT growth; FeCl3 solution was deposited onto the surface of graphene/Cu substrate by spin-coating method. The density and quality of the CNTs can be controlled by varying the concentration of FeCl3 salt catalyst. Although the aforementioned methods are great advancements in CNGHTF production, they still require multiple steps of fabrication/synthesis and have difficulty ensuring direct bonding between graphene and CNTs.
Figure 5. Schematic illustration of fabricating of a CNT–graphene hybrid film via rapid heating and cooling CVD, and the demonstrating SEM images [33].
Download figure:
Standard image High-resolution image2.4. One-step growth of CNGHTFs by chemical vapor deposition
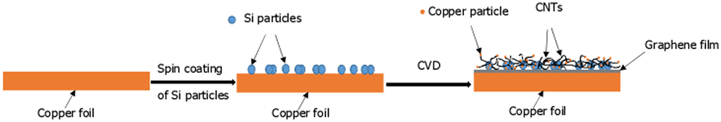
CVD presents a promising manufacturing technique for mass production of CNTs, especially producing large yields of substrate-free CNTs. Generally, CVD is based on the decomposition of carbon-containing gases on catalysts in the form of thin films or as-deposited nanoparticles (usually transition metals, i.e. Fe, Co, Ni). CVD has been sucessfully applied for controlled growth of graphene and CNTs [76]. Recently, many attempts have been made to prepare CNGHTFs via simultaneously joining the as-precipitated graphene with pre-coated CNTs on the surface of catalysts such as Ni, Fe [46]. For example, Chen et al [46] reported a highly conductive graphene–CNT hybrid material prepared using silicon nanoparticle (Si NP)-coated copper foil and a one-step CVD process. The shape and property of the hybrids can be tuned by changing the growth conditions (e.g., temperature and growth time) and the size, density and pattern of Si NPs. Figure 6 shows a schematic of one-step growth of a graphene–CNT hybrid material on Si NP pre-coated copper foil by CVD method under atmospheric pressure. Clearly, a major disadvantage of the CVD-based approach is the required use of high growth temperatures (associated with high power consumption), explosive gases and toxic chemicals for the growth of both CNTs and graphene nucleation.
Figure 6. Schematic of one-step growth of a graphene–CNT hybrid on Si NPs pre-coated copper foil by CVD method.
Download figure:
Standard image High-resolution image2.5. Modified CVD methods via thermal decomposition of carbon source on catalyst surfaces
Previously, CNGHTFs were grown by using the starting materials of solution-based graphene or chemically derived layered graphene, followed by CNT growth using a CVD process. This technique is obviously preferred for successful incorporation with CNTs. However, this method's disadvantages of long processing times and high growth temperatures limit its practical application at industrial scale. Therefore, it is highly imperative to develop alternative fabrication methods that are time- and power-saving, environmentally friendly, and especially compatible with the processing of CNT–graphene hybrids. In this regard, modified CVD methods have been recently proposed to reduce processing time and/or lower the growth temperature, in combination with reduction of electric power by direct heating of carbon source on catalytic substrates/surfaces. Using this concept, Nguyen et al [27] reported a versatile method based on low vacuum annealing of cellulose acetate on nickel (Ni) surface for rapid fabrication of graphene and CNT–graphene hybrid films with tunable properties (figure 7). Uniform films mainly composed of tri-layer graphene can be achieved via the surface precipitation of dissociated carbon at 800 °C for 30 s under vacuum conditions of ∼0.6 Pa. The surface precipitation process is further found to be efficient for joining the precipitated graphene with CNTs pre-coated onto the Ni surface, consequently generating the hybrid films. Remarkably, the carbon precipitation process was found to be effective in fabricating CNT–graphene hybrid films with improved properties. The annealing and/or cooling processings of precipitated carbon atoms resulted in forming graphene on CNT-free areas of the Ni surface via crystalization. Simultaneously, such atoms precipitating under or near CNTs are preferentially filled into defective sites of the CNTs, creating C–C links between CNTs and the adjacent precipitated graphene, that is, constructing the CNT–graphene hybrids. The precipitated graphene sheets in the resultant hybrid film serve as an interfacial layer for reducing contact resistance between the CNTs and Ni substrate, resulting in improved field electron emission performance, as shown in figure 8.
Figure 7. Schematic presentation of a rapid fabrication of transparent conductive CNT–graphene hybrid films via a low vacuum annealing of cellulose acetate on nickel (Ni) surface [27].
Download figure:
Standard image High-resolution imageFigure 8. SEM image of C–C links between CNTs and the adjacent precipitated graphene, and field emission current density on the applied electric field (J–E) for the CNTs (black squares) and its hybrid films (red circles) [27].
Download figure:
Standard image High-resolution imageUsing another way—adjusting the growth time, C concentration, and microwave power—Deng et al [31] sucessfully performed catalyst-free and self-assembled growth of graphene flakes (GFs) on CNT arrays and applied them for high-performance field emission. In this approach, the deposited C atoms diffuse on the surface of CNTs and aggregate by means of forming covalent bonds at the intrinsic defects of CNTs, resulting in the nucleation of GFs. Similarly, by carefully selecting metal-based catalysts, Zhu et al [25] successfully fabricated CNT–graphene hybrids via syngas production. The authors turned the undesired carbonaceous materials in the production process into valuable hybrids of carbon nanomaterials using 2Cr-Ni as the substrate. Ni nanoparticles interacted with the CeO2 support by absorbing large amounts of microwave energy. The generating temperatures were high enough to promote the growth of cup-stacked CNTs and the formation of multi-walled CNT–graphene hybrid via thermal decomposition of carbon source onto either a metal or a zeolite catalyst. Notably, these approaches showed excellent processability of hybrid CNT–graphene films and significant improvements in the electrical or optical properties that would be necessary for practical applications in the future.
In another work Cao et al [74] reported a successful strategy to directly assemble hybrid CNT–graphene films by a blown bubble method combined with selective substrate annealing. In this method, PMMA serves as the carbon source for growing single to few-layer graphene among the CNT networks until a continuously hybridized structure is formed. The authors also built a Si-based solar cell using a MWCNT–graphene electrode, in which the graphene hybridization provides an effective means of optimizing the structure and improving the performance of the cell. The received results indicate that it is an efficient strategy for producing a variety of hybrid CNT–graphene films via thermal decomposition of carbon source on catalyst surfaces. Moreover, the strategy is also effective in the application of Si-based solar cells with MWCNT–graphene electrodes, in which the graphene hybridization provides effective means for structural optimization and enhanced performance of the cell.
3. CNGHTFs—applications
CNGHTFs have been utilized for various applications, such as transparent conductors and electron field emitters [31–34], field-effect transistors [35, 36], sensors [37–41] especially supercapacitors [42, 43].
3.1. CNGHTFs in transparent conductions and electron field emitters
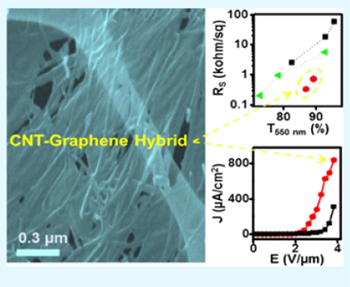
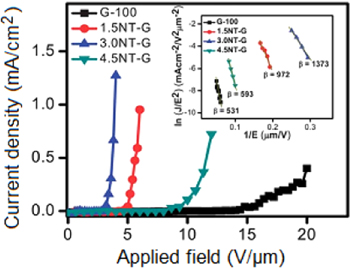
CNGHTF materials and their transparent thin films have a profound impact in transparent conductions and electron field emitters [31–34]. Rodney et al [34] sucessfully fabricated CNGHTFs on glass or Si/SiO2 substrates. Pure CNT sheets exhibit optical transmittance for unpolarized light T550(u-p) of approximately 85% and a difference between perpendicular (⊥) and parallet (||) light components (T550(⊥)–T550(||)) of approximately 14%. Meanwhile, the difference for the CNT–graphene and graphene–CNT films is in the range of 11–13% and 4–6% respectively. Compared with single layer graphene and pure CNTs, CNGHTFs have lower sheet resistances. Indeed, pure CNT films have higher anisotropy (Rs(||) ~ 710 Ω sq−1 and Rs(⊥) ∼ 1000 Ω sq−1) as compared to Rs(||) ∼ 220 Ω sq−1, Rs(⊥) ∼ 530 Ω sq−1 for graphene–CNT hybrid films, and Rs(||) ∼ 340 Ω sq−1, Rs(⊥) ∼ 800 Ω sq−1 for CNT–graphene hybrid films. Nguyen et al [33] prepared and controlled the thickness of CNGHTFs using a facile CVD method that used iron films and C2H2 gas as the catalyst and carbon feedstock, respectively. Compared with pristine graphene film, CNGHTFs showed significant increase in sheet resistance to 420 kΩ sq−1 and presented an optical transmittance of 72.9%. The hybrids exhibited excellent field emission properties, superior than pristine CNT arrays and graphene films with a low turn-on electric field of 2.9 V μm−1 and a threshold electric field of 3.3 V μm−1 (figure 9). By using microwave PECVD, Deng et al [31] demonstrated a catalyst-free, self-assembled and vapor-solid approach to synthesis of CNGHTFs. Compared with pristine CNT arrays, CNGHTFs showed an excellent field emission with a low turn-on electric field of 0.73 V μm−1 and a threshold field of 1.16 V μm−1. These results suggested that the CNGHTFs are promising materials for the development of transparent conductors and electron field emitters.
Figure 9. Field emission current density versus applied field of graphene and CNT–graphene with different CNT densities. The inset shows their corresponding Fowler–Nordheim plots [33].
Download figure:
Standard image High-resolution image3.2. CNGHTFs in field-effect transistors
Using CVD method, Zhang et al [35] successfully synthesized long–aligned SWCNTs, which crossed the gap between rGO electrodes on Si/SiO2 substrates. The effective mobility (μ) of CNTs–rGO was estimated to be 748 cm2 V−1 s−1. In addition, Myung et al [36] synthesized CNGHTFs on copper foil by thermal CVD method. After the synthesis of CNGHTFs, a graphene-based hybrid material was transferred onto Si/SiO2 and polyethylene terephthalate (PET) substrate. Compared with graphene and SWCNT FETs, the CNGHTF FETs had higher field-effect mobilities. The field-effect mobilities of SWCNTs, graphene, and SWCNTs-graphene FETs were 58.78 ± 36.17, 341.7 ± 259.4, and 394.46 ± 176.27 cm2 V−1 s−1 respectively. After the formation of SWCNTs-graphene, the electrical conductivity of this hybrid dramatically increased. The results suggest that CNGHTFs can be applied to fabricate flexible and transparent electrodes on PET films.
3.3. CNGHTFs in biosensors
CNGHTFs provide an increase in surface area for enzyme immobilization and also create 3D conductive networks for efficient electron transfer to surrounding enzyme molecules [40]. Unnikrishnan et al [37] fabricated a simple electrochemical sensor based on rGO–CNT modified GC electrode for the sensitive determination of carbamazepine (CBZ). The rGO–CNT composite films- based sensors possessed excellent analytical parameters, with a sensitivity of 5.1076 μA μM−1 cm−2 in high linear range from 50 nM to 3 μM, low detection limit of 29 nM. Chen et al [38] also fabricated high-performance electrochemical sensors based on CNGHTFs modified GC electrodes for acetaminophen detection. The sensors based on CNGHTFs exhibited a wide linear range of 0.05–64.5 μM, a low detection limit of 38 nM, and good selectivity and stability. Mani and co-workers [39] constructed an rGO–CNT nanocomposite-based hybrid using electrochemical platform for detection of glucose, based on oxygen consumption at the cathode. The proposed biosensor presented a wide linear range of 0.01–6.5 μM and a low detection limit of 34.7 nM. Thakoor et al [41] fabricated CNGHTFs by a thermal CVD method. The CNGHTFs were transferred from copper foil to the GC electrodes for measurement of glucose concentration. The hybrid film modified electrodes exhibited high ability towards the glucose determination with excellent analytical parameters, such as the high sensitivity of 19.31 μA mM−1 cm−2 and the low detection limit of 0.5 mM in a linear range of 2–8 mM.
3.4. CNGHTFs in supercapacitors
Due to their high surface area, conductivity, electrochemical stability, elasticity and mechanical stability, CNGHTFs are considered as attractive materials for supercapacitor applications. Kim et al [42] fabricated CNT–graphene composite film based supercapacitors with capacitance of 653.7 μF cm−2 at 10 mV s−1, which is considerably higher than the value of 99.6 μF cm−2 at 10 mV s−1 for graphene-only electrodes. The hybrid electrode maintained a high capacitance of 490.3 μF cm−2 at the charging and discharging rates of 300 mV s−1, indicating that it is a potential supercapacitor with greater capacitance than that of graphene-only electrodes. Cui and co-workers [43] fabricated supercapacitors based on rGO–CNT. Compared with rGO in both positive and negative potential windows, the galvanostatic charge/discharge curves of rGO–CNT electrodes showed more symmetric and triangular shapes. These rGO–CNT electrodes showed the higher specific capacitance as compared to rGO. Introducing a small amount of CNTs into rGO sheets, the capacitance of rGO–CNT hybrid had a negative potential window of 237 F g−1, which was about twice that of rGO only (∼95 F g−1) at the scan rate of 50 mV s−1. An increasing the amount of CNTs can significantly enhance the capability and cyclic stability rates of the electrode. CNGHTF-based supercapacitors have been demonstrated as promising candidates for use in hybrid vehicles and electrical vehicles.
4. Conclusion and future prospects
A wide variety of fabrication methods have been used to produce CNGHTFs by combining CNT (1D) and/or graphene (2D) into the novel 2D or 3D sp2-hybridized carbon structures, via inducing intermolecular junctions between the individual nanotubes and graphene sheets. rebar graphene has shown great potential as a material for research on planar hybrid sheets structures in the future. However, figuring out a simple-scalable process to produce a controllable construction of CNGHTFs using a one-pot process is still a challenging research area. We present various applications of the hybrid materials, such as electron field emitters, field-effect transistors, biosensors and supercapacitors. In most cases, CNGHTFs showed superior performances than those of pristine GO/graphene or CNT materials. Therefore, the CNGHTFs exhibit as high-potential materials for various practical applications. Advanced—in situ—characterizations will be necessary for full understanding of the growth mechanisms, but this aspect is not presented in this review. Studies on the interactions between gases, catalysts, and boundaries between CNTs and graphene are needed for a deeper understanding of 2D or 3D sp2-hybridized-based architectures, which are the building blocks of all carbon-based devices.
Acknowledgments
This research is funded by Vietnam National Foundation for Science and Technology Development (NAFOSTED) under grant number 103.02-2014.68 and the Vietnam Academy of Science and Technology (VAST) under grant numbers VAST.HTQT.NGA.10/16-17, VAST 03.06/14-15 and VAST 03.01/15-16. We also acknowlege Acad. Nguyen Van Hieu and Dr Nguyen Bich Ha for their valuable suggestions and discussions.