Abstract
One-dimensional self-assembled single-crystalline hexagonal tungsten trioxide (WO3) nanostructures were synthesized by wet chemical-assisted hydrothermal processing at 120 °C for 24 h using sodium tungstate and hydrochloric acid. Urchin-like hierarchical nanorods (petal size: ∼16 nm diameter and 110 nm length) were obtained. The samples were characterized by field emission scanning electron microscopy, transmission electron microscopy, energy dispersive x-ray spectroscopy and x-ray diffraction. Sensors based on WO3 nanorods were fabricated by coating them on SiO2/Si substrate attached with Pt interdigitated electrodes. NH3 gas-sensing properties of WO3 nanorods were measured at different temperatures ranging from 50 °C to 350 °C and the response was evaluated as a function of ammonia gas concentration. The gas-sensing results reveal that WO3 nanorods sensor exhibits high sensitivity and selectivity to NH3 at low operating temperature (50 °C). The maximum response reached at 50 °C was 192 for 250 ppm NH3, with response and recovery times of 10 min and 2 min, respectively.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 3.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
In recent years one dimension (1D) nanostructures, such as tungsten oxide nanowires (NWs) or nanorods (NRs) have attracted special attention due to their specific properties which are expected to be linked to their anisotropic shape related to a large surface to volume ratio. Gas sensors based on 1D nanostructures have advantages of higher sensitivity, superior spatial resolution and rapid response owing to the high surface to volume ratio compared to thin film gas sensors. Tungsten oxide NRs have shown interesting properties as NO2 [1–3], CO [4], H2 [5, 6], C2H5OH [7, 8], NO [9], O3 [10], H2S [11] sensors. On the other hand, tungsten oxide (WO3) is an important n-type semiconducting material with a band gap of 2.7 eV, finding applications in gas sensing, photocatalysis and electrochromic devices [12]. WO3 NRs with hexagonal structure were synthesized by hydrothermal methods [13–16] for their advantages such as simple operation, low-cost, potential for large scale production and forming crystalline structure with good stability. Simple and low cost methods for well-ordered nanostructure construction are preferred in gas-sensing and other commercial applications. In the present paper, we deal with detailed NH3 sensing properties of WO3 NRs synthesized by hydrothermal process. Ammonia (NH3) is a typical reducing gas. As a toxic but colorless gas with a special odor, it is a major air pollutant emitted from agricultural practices. The need to detect low ammonia concentrations has greatly increased in many fields of technological importance, such as food technology, chemical engineering, medical diagnosis, environmental protection, monitoring of car interiors and industrial processes. The electrical properties of the NRs can change upon exposure to gas and can be restored upon re-exposure to air at low temperature. Such low temperature-processes are very desirable in detecting toxic gases safely at room temperature.
2. Experimental
All the chemical reagents were purchased from commercial sources of analytical grade and used without further purification. Sodium tungstate dihydrated powder (Na2WO4.2H2O) was used as tungsten source. In a typical experiment, 4.125 g Na2WO4.2H2O was dissolved into 12.5 ml of bi-distilled water and stirred for 30 min to form a translucent 1 M Na2WO4 solution. Then, a 3 M HCl solution was added drop wise under stirring in succession to acidify the Na2WO4 solution to a pH of 1.8 to 2.2. The mixed reaction system was stirred for 4 h before introducing the obtained solution in a 20 ml teflon-lined stainless steel autoclave and the temperature was set at 120 °C for 24 h under autogenous pressure. The pH of the solution remained close to 2 during the whole synthesis. The obtained powder was washed several times with distilled water and ethanol to remove possibly residual ions, and then dried at 80 °C for 24 h in air. Finally, the dried WO3 powders were subjected to heat treatment at 400 °C for 1 h.
Morphology, composition and phase of the samples were characterized by field emission scanning electron microscopy (FE-SEM), transmission electron microscopy (TEM), energy dispersive x-ray spectroscopy (EDX) and x-ray diffraction (XRD).
The obtained powders were dispersed in mixture of ethanol and polyethylene glycol solution using sonication to make a slurry. After that the slurry was coated onto Pt-interdigitated electrodes with the gap between the fingers of about 30 μm by the spin-coating method and then dried in air at 80 °C for 24 h. The sensor sample was annealed at 400 °C for 2 h to evaporate organic species and to stabilize the materials structure. The electrical resistance of the samples at different temperatures was measured as a function of the NH3 concentration ranging from 25 ppm to 250 ppm. The sensor was placed in a glass test chamber and heated by an external source through the sample holder. The operating temperature of the sensor was determined with a thermocouple attached near the sensor element and varied from 50 °C to 350 °C. The response of the sample to ammonia gas was thus measured in a static system. The resistance between the two electrodes was measured at an applied voltage of 5 V. Herein, the sensor response to NH3 gas was defined according to the standard definition of reducing gas as the ratio S = Rg/Ra for operating temperature below 150 °C and S = Ra/Rg for operating temperature above 150 °C, where Ra is the sample resistance in air and Rg is its resistances in the presence of NH3 gas. The sensor response dynamics were evaluated through the response time (τres), calculated as the time duration for sensor resistance to reach 90% of its steady-state value during the exposure, and recovery time (τrec) as the time needed to recover 90% of the original baseline resistance.
3. Results and discussion
3.1. Crystal structure and surface morphology
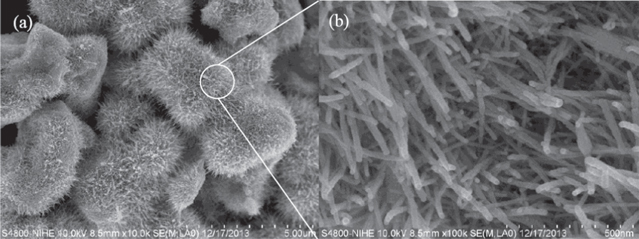
Figure 1 presents a typical SEM image of the NRs grown by hydrothermal process. Most of the nanostructures are single NRs, several NRs grown side by side. Besides the rod-like nanostructures, no other products can be found. The WO3 NRs are straight and uneven surface with uniform dimension along their axial direction over a large area. TEM image shown in figure 2 exhibits the rough surface of the NRs with large specific surface area, which is consistent with the SEM image in figure 1. The average diameter and length of as-prepared WO3 nanorods are estimated to be ∼16 nm and 110 nm, corresponding to an aspect ratio of ∼7.
Figure 1. FE-SEM images of WO3 NRs with different magnifications of 10 000 (a) and 100 000 (b).
Download figure:
Standard image High-resolution imageFigure 2. TEM image of as-synthesized WO3 NRs.
Download figure:
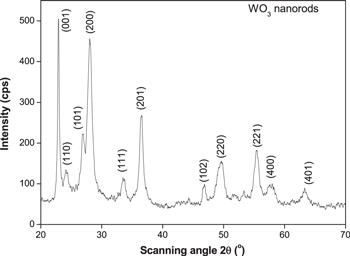
Standard image High-resolution imageFigure 3 shows the XRD pattern of WO3 NRs. All the diffraction peaks of pre-annealed WO3 can be indexed to hexagonal structure WO3 (h-WO3) with lattice constants of a = b = 0.7298 nm, c = 0.3899 nm, α = β = 90°, γ = 120° (JCPDS Card No. 75-2187), space group of primitive cell is P6/mmm [17]. No peaks of any other phase or impurities were observed from the XRD patterns. Strong and sharp diffraction peaks also indicate a good crystallinity of the sample. The crystallite size of the sample calculated from Debye–Scherrer's equation equals to about 20 nm. This result is roughly in agreement with the TEM measurement (figure 2).
Figure 3. XRD pattern of as-synthesized of WO3 NRs.
Download figure:
Standard image High-resolution image3.2. Chemical composition
The EDX spectrum, acquired from individual NRs as shown in figure 4, indicates that tungsten and oxygen are the major elements present in the NRs with a 3:1 molar ratio for oxygen and tungsten elements, which solely constitute the composition of the h-WO3 NRs. The C signal shown in the spectrum is the graphite layer deposited onto WO3 powder before EDX analysis. The Na content is small and comes from traces of the precursor remaining in the product.
Figure 4. EDX spectrum of WO3 NRs.
Download figure:
Standard image High-resolution image3.3. NH3 sensing property
Figure 5 shows the dynamic sensing characteristic of bare WO3 NRs gas sensor towards different NH3 gas concentrations at operating temperature of 50 °C. As observed, the sensor resistance increased gradually when NH3 gas was introduced into the chamber and then increased slowly to reach a stable state. The response of the sensor increases with increasing ammonia concentration from 25 ppm to 250 ppm. When the gas was turned off, the resistance dropped sharply and then reached its initial value. This increase of the resistance upon exposure to a reducing gas indicated p-type conduction of WO3 at 50 °C. The p-type behaviour of h-WO3 at low temperature and the conversion of p-type to n-type conductivity in WO3 by changing the operating temperature were also reported by other authors [18, 19]. The origin of the n- to p-type transition is related to the formation of an inversion layer at the surface of the WO3 NRs at room temperature. When the WO3 NRs sensor is exposed to air, the adsorption of a large number of oxygen and water molecules creates a p-type surface inversion layer. With increasing temperature the semiconductor surface becomes first intrinsic because of water and oxygen desorption, and then n-type depleted surface at high temperature due to further desorption.
Figure 5. Dynamic response of the pure WO3 NRs gas sensor towards NH3 at operating temperature of 50 °C.
Download figure:
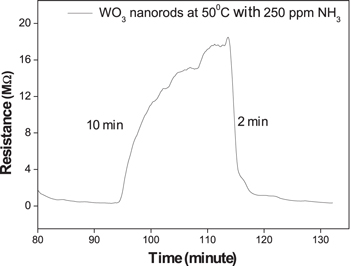
Standard image High-resolution imageFigure 6 shows enlarged part of the data of figure 5 measured at 250 ppm NH3 to reveal the moments of gas input and gas stop. The bare WO3 NRs showed sensitivity of 192 at a NH3 concentration of 250 ppm. The response and recovery times to 250 ppm NH3 are estimated to be 10 min and 2 min, respectively. The response rate was slow due to low working temperature.
Figure 6. The enlarged part of the response curve in figure 5 to 250 ppm NH3.
Download figure:
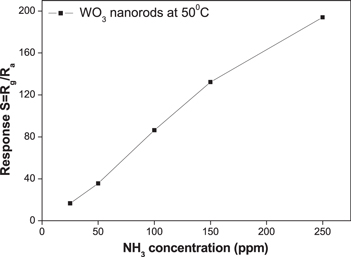
Standard image High-resolution imageThe relationship between WO3 NRs sensor response and NH3 concentration was determined at the operating temperature of 50 °C. The sensitivity was defined as the ratio of the sensor resistance at various given ammonia concentrations to that in ambient atmosphere. The high sensitivity, low detection level and large range of ammonia concentration of this sensor are very interesting points for its development. The resistance and response change linearly with NH3 concentration (figure 7). Linear dependence of response on gas concentration is an advantage for designing readout signal circuits. The detection limit of NH3 for bare WO3 NRs sensor is estimated to be 25 ppm at the operating temperature of 50 °C. It is thus useful for low-power gas-sensing application, and low concentration ammonia detection is actually desired for the development of highly sensitive sensor. In addition, the WO3 NRs sensor prepared by this method can detect NH3 at relatively low operating temperature with high gas response compared to some other methods. To the best of our knowledge, there have been few reports on the NH3 sensing at near room temperature of WO3 nanomaterials [20]. The nanowire-like structure of tungsten oxide synthesized by the deposition of tungsten metal on the substrate of porous single-wall carbon nanotubes film, followed by thermal oxidation process, has a maximum response of 2.39 toward 300 ppm NH3 at 250 °C [21].
Figure 7. Ammonia sensitivity of WO3 sensor at temperature of 50 °C.
Download figure:
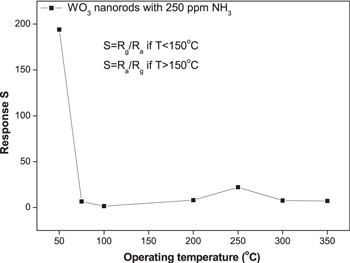
Standard image High-resolution imageWe also measured the sensor response to ammonia gas at different temperatures of 75, 100, 200, 250, 300, 350 °C to examine the effect of operating temperature on the sensitivity to NH3 gas. Figure 8 shows the response of WO3 NRs based sensor as a function of ammonia concentration operating at different temperatures. The response of the sensor to NH3 increased monotonously with increasing gas concentration. The sensor response increases with increasing operating temperatures from 100 °C to 250 °C and then decrease as the operating temperature increases further. At 250 °C, the sensor response in the presence of 50 ppm NH3 gas was 7, while it increased to 22 for NH3 concentration of 250 ppm. At low operating temperatures (100 °C – 250 °C), sufficient thermal energy is essential to overcome the activation energy barrier, however, when the operating temperature is too high (above 250 °C), the desorption process becomes dominant. Furthermore, the diffusion depth becomes lower at a high temperature [22]. Thus, the optimum operating temperature of WO3 NRs gas sensor in the low temperature region and in the high temperature region are around 50 °C and 250 °C, respectively (figure 9). So, nanorod-like WO3 nanostructures were particularly suitable for ammonia detection at these temperatures and concentrations.
Figure 8. The response of WO3 NRs gas sensor to different concentrations of NH3 at different operating temperatures.
Download figure:
Standard image High-resolution imageFigure 9. The response of WO3 NRs to 250 ppm NH3 at different operating temperatures.
Download figure:
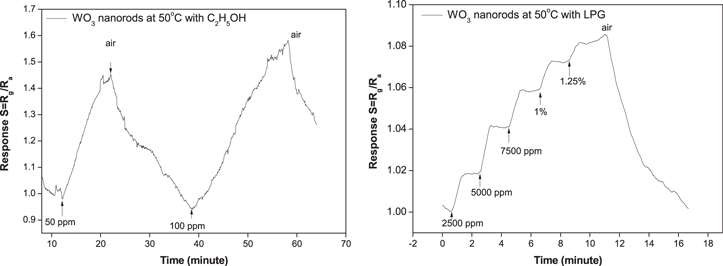
Standard image High-resolution imageIn order to investigate the selectivity of bare WO3 NRs gas sensor, the sensor was exposed to other reducing gases such as C2H5OH (ethanol) and LPG (liquefied petroleum gas) at various concentrations. Ethanol gas response of bare WO3 NRs sensor at the operating temperature of 50 °C is displayed in figure 10. It can be seen from figures 5 and 10 that pure WO3 NRs sensor exhibits a very impressive response toward NH3 but is almost insensitive to ethanol (50 to 100 ppm) and low sensitivity to LPG at concentrations ranging from 2500 ppm to 1.25% at the same conditions. These results indicated fairly good ammonia selectivity of the WO3 NRs thin films.
Figure 10. Dynamic response of the pure WO3 NRs gas sensor toward C2H5OH (left) and LPG (right) at operating temperature of 50 °C.
Download figure:
Standard image High-resolution image4. Conclusion
In summary, WO3 NRs were synthesized through soft chemical process assisted by hydrothermal technique at 120 °C for 24 h with the pH value of reaction solution of 1.8 to 2.2. The obtained urchin-like products consisted of many WO3 NRs which are rather uniform with diameters in the range of 15–20 nm and lengths of 100–150 nm. As a potential gas sensor, the WO3 NRs exhibit high response and selectivity toward NH3 gas at near room temperature. The sensitivity of the WO3 NRs to 250 ppm NH3 gas is about 192 at an operating temperature of 50 °C.
Acknowledgments
This research is funded by Vietnam National Foundation for Science and Technology Development (NAFOSTED) under grant number 103.99-2012.31.