Abstract
Normally in proton exchange membrane water electrolysis (PEMWE), the anode has the largest overpotential at typical operating current densities. By development of the electrocatalytic material used for the oxygen evolving electrode, great improvements in efficiency can be performed. In electrochemistry, rare metallic oxides RuO2 and IrO2 exhibit the best catalytic properties for the oxygen evolution reaction (OER) in acid electrolytes compared to other noble metals. RuO2 is the most active catalyst and IrO2 is the most stable catalyst. An oxide containing both elements is therefore expected to be a good catalyst for the OER. In this study IrxRu1−xO2 nanosized powder electrocatalysts for oxygen evolution reaction is synthesized by hydrolysis method. Cyclic voltammetry, anodic polarization and galvanostatic measurements were conducted in solution of 0.5 M H2SO4 to investigate electrocatalytic behavior and stability of the electrocatalyst. The mechanisms of the thermal decomposition process of RuCl3.nH2O and IrCl3.mH2O precursors to form oxide powders were studied by means of thermal gravity analysis (TGA). X-ray diffraction (XRD) and scanning electron microscopy (SEM) were used analysis for determination of the crystallographic structure, morphology and catalysts particle size. Based on the given results, the IrxRu1−xO2 (x = 0.5; 0.7) compounds were found to be more active than pure IrO2 and more stable than pure RuO2.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 3.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Since depletion of fossil energy sources, the energy demand for developing countries and the environmental pollution caused by greenhouse gas emissions, etc, the development of renewable energy sources has become imperative. Hydrogen can be used like a conventional fuel: to be burned using air in engines, turbines or boilers to provide energy, or converted to electricity directly without the thermodynamic limitations of a thermal process in low temperature proton-exchange membrane fuel cells (PEMFC). Water electrolysis is one of the few processes where hydrogen can be produced from renewable energy sources such as photovoltaic or wind energy without evolution of CO2. In particular, proton exchange membrane water electrolysis (PEMWE) is considered as a promising methodology for producing hydrogen as an alternative to the conventional alkaline water electrolysis. A PEM water electrolyser possesses certain advantages compared with the classical alkaline process in terms of simplicity, pure products, high energy efficiency and specific production capacity. This method uses electricity to split the pure water into hydrogen at cathode and oxygen at anode. There are a lot of intensive researches and developments which have been done on PEMWE and commercialized products were provided by global companies such as Hamilton Sundstrand in USA, Htec in Germany and RRC 'Kurchatov Institute' in Russia. However, high cost of investment using precious and expensive catalysts material has limited their mass commercialization [1, 2]. In addition, the overpotential loss on anode of PEMWE for oxygen evolution reaction (EOR) is still relatively high and this reduces the efficiency of water electrolysis processes. Therefore, recent PEMWEs research has been focused on new catalysts material in order to improve the anode active surface area, catalyst utilization, and stability by using nanosized powder materials [3–10].
For the oxygen evolution reaction OER in acidic electrolytes noble metal oxide catalysts are the most active and stable. RuO2 is more active than IrO2, but it undergoes heavy erosion during OER and loses its activity. In contrast, IrO2 is more stable than RuO2 but it is very expensive because of being one of the rarest elements in the Earth's crust [1]. Therefore, a compound of the formula IrxRu1−xO2 would be a more appropriate electrocatalyst for OER in acidic electrolyte due to its considerable activity, stability and lower cost. Ru–Ir binary oxides via thermal decomposition were fabricated and it was found that the Ru0.3Ir0.7O2 electrode demonstrated maximum electrocatalytic activity [11]. Mattos-Costa et al [12] prepared IrxRu1−xO2 coatings on Ti plates using the sol–gel method and observed the 'best' performance for the Ru0.7Ir0.3O2 coating. In the present work we synthesized IrxRu1−xO2 (x = 0.5, 0.7) nanosized powder electrocatalysts for oxygen evolution reaction by hydrolysis method. Physical and electrochemical cheracterization of catalysts were measured and the affects of the content of Ru on performance of IrxRu1−xO2 mixture catalysts were also discussed.
2. Experimental
2.1. Metal oxide powder catalyst preparation
The IrxRu1−xO2 oxide powder catalysts were prepared by hydrolysis method in alkali medium. At first, RuCl3.nH2O (99.98% trace metals basis, Aldrich), IrCl3.mH2O (99.98% trace metals basis, Aldrich) were dissolved in deionization water with the exactly calculated metal precursors. The aqueous solution was then heated (100 °C) under air atmosphere and magnetically stirred for 1 h. After, sodium hydroxide (1 M) was added to the solution in order to obtain the precursor-hydroxide. This mixture was maintained under stirring and heat (100 °C) for 45 min. The given precipitate was then filtered and washed with deionization water. After washing, the precursor-hydroxide was dried for 5 h at 80 °C. Finally, the dried paste was calcined in air at 450 °C for 1 h with a heating ramp of 5 °C min−1. The IrxRu1−xO2 oxide catalyst powders were synthesized with the various content of Ir (x = 0, 0.5, 0.7, 1).
2.2. Physical cheracterization of oxide powder catalysts
The mechanisms of the thermal decomposition process of RuCl3.nH2O and IrCl3.mH2O precursors to form oxide powders were studied by means of thermal gravity analysis (TGA) in air from ambient temperature to 700 °C. The physical phase and structure of the oxide powder catalysts were determined by x-ray diffraction (XRD) measurements using a SIEMENS D5000 with CuKα radiation (λCu-Kα = 0.154 nm) powered at 45 kV. The surface morphology and particle size of the catalysts materials were examined by scanning electron microscopy (SEM). This apparatus was equipped with a HITACHI S-4800. Both methods were done at Institute of Materials Science.
2.3. Electrochemical cheracterization of oxide powder catalysts
To investigate the activity and stability of the electrocatalyst powders, the electrochemical measurements such as cyclic voltammetry, polarization and galvanostatic were conducted in solution of 0.5 M H2SO4. All measurements were carried out in a standard three-electrode cell with working area controlled around 1 cm2. A Pt foil served as counter electrode and a saturated calomel electrode (SCE) was used as reference electrode, the equipment was PARSTAT-2273 (EG&G-USA).
The catalyst layer was deposited on the carbon paper (AvCarrb 1071-USA) with electrocatalyst loading of 4 mg cm−2. To prepare the thin-film electrocatalyst layers, a homogeneous ink composed 7.00 mg of powder catalyst, 15 μL of nafion solution (5 wt%), 1 ml of isopropanol and 1 ml of deionization water were homogenized by stirring for 30 min then mixing in an ultrasonic bath for 30 min. The mixture (50 μL) was dropped by brushing on the cacbon paper surface and drying in air. This process was repeated until reaching sufficient electrocatalyst loading.
3. Results and discussions
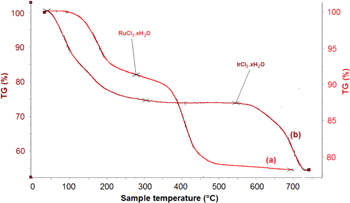
Figure 1 shows TGA diagrams of RuCl3.nH2O (a) and IrCl3.mH2O (b) salts. For RuCl3.nH2O, due to the salt being hydrated at low temperatures (<250 °C) there is a considerable decrease in the TGA curve. This may correspond to a revolution of physical and chemical water in salt molecule. When temperature is higher than 300 °C, the RuCl3 is initially decomposed, which leads to a decline in the TGA curve. The decomposition process is completely finished at 500 °C and it is clear to observe the TGA curve becomes stable. Therefore, the suitable temperature for RuO2 decomposition in hydrolysis process may be in a range 300–500 °C. For IrCl3.mH2O salt, the water release process is finished at temperature 303 °C and on the chart, the TGA curve drop slowly and regularly meaning that the bonding between physical and chemical water and salt molecule is quite strong. After releasing water, there is a stable stage on TGA curve and the composition process begins at temperature 450 °C. The decrease of salt in weight is finished completely at temperature 725 °C. Therefore, it may be suitable to synthesis the catalyst powder IrO2, Ir0.7Ru0.3O2, Ir0.5Ru0.5O2, RuO2 by hydrolysis process.
Figure 1. TGA diagrams of precursors (a) RuCl3.nH2O and (b) IrCl3.mH2O.
Download figure:
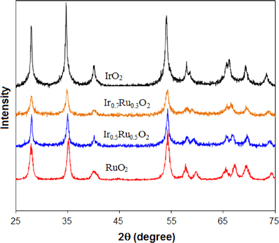
Standard image High-resolution imageFigure 2 shows XRD patterns of IrxRu1−xO2 oxide catalyst powders with x = 0, 0.5, 0.7, 1 which are synthesized at temperature 450 °C. As can be seen in the figure, diffraction peaks of pure ruthenium oxide and iridium oxide are similar and all peaks match a rutile-structure as indexed. The diffraction peaks of ruthenium oxide appear at higher 2θ values compared to the peaks of iridium oxide. For mixture oxide powders, XRD pattern also confirms a rutile structure. There is not the specific diffraction peak of ruthenium oxide and the 2θ of peaks for the mixture oxides are shifted to higher values. When increasing the content of Ir in the mixture, the XRD peaks are close to pure iridium oxide. Therefore, when inserting RuO2 the parameter of lattice IrO2 is changed slightly in the mixture and ruthenium oxide and iridium oxide form a solid solution
Figure 2. XRD patterns of IrxRu1−xO2 with x = 0; 0.5; 0.7; 1 oxide powder electrocatalysts.
Download figure:
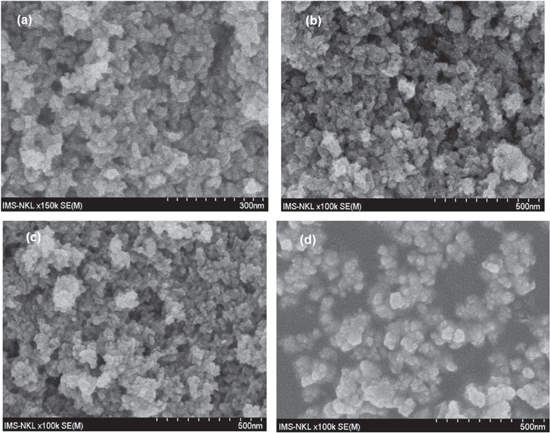
Standard image High-resolution imageFigure 3 presents morphology of catalyst oxide powders captured by SEM. All powder samples are small and uniform in size. SEM measurements showed that the synthesized powder catalysts are composed of aggregated nanoparticles. The IrO2 have the largest size (<50 nm) while the size of RuO2 and the mixtures is smaller than 30 nm. Due to small size, the catalyst powders have high active area meaning high activity for electrochemical reactions in PEMWEs.
Figure 3. SEM pictures of IrxRu1−xO2 electrocatalytic powers. (a) x = 0, (b) x = 0.5, (c) x = 0.7, (d) x = 1.
Download figure:
Standard image High-resolution imageThe cyclic voltammetric curves (CV) of catalyst powders with various contents of Ir in solution of 0.5 M H2SO4 are shown in figure 4. The CV curves show the different redox processes occurring on the surface of the catalysts. The peaks of pure IrO2 observed at about 0.6 and 1.2 VSCE are attributed to the redox transitions of the Ir(III)/Ir(IV) and Ir(IV)/Ir(VI) surface oxyiridium groups, respectively, while the peaks of pure RuO2 observed at about 0.35 and 0.95 VSCE are attributed to the redox transitions of the Ru(III)/Ru(IV) and Ru(IV)/Ru(VI) surface oxyruthenium groups, respectively. The voltammogram of pure RuO2 is considerably higher in comparison with voltammogram of IrO2. The voltammetric curve of Ir0.5Ru0.5O2 and Ir0.7Ru0.3O2 mixture powders is similar to the curves of pure oxides. However, when the content of Ru is increased, the voltammograms of mixtures are close to voltammogram of RuO2. This may imply that the activity of the mixture catalyst is increased in the presence of Ru.
Figure 4. Cyclic voltammograms of IrxRu1−xO2 electrocatalytic powers in solution of 0.5 M H2SO4; scanning potential velocity 50 mV s−1.
Download figure:
Standard image High-resolution imagePolarization measurements of oxide powders samples in 0.5 M aqueous H2SO4 are shown in figure 5. The electrochemical parameters estimated from these curves are summarized in table 1. RuO2 is the most active for oxygen revolution reaction because its potential of oxygen evolution is the lowest. With increasing content of Ru in the powder mixtures, the catalytic properties are optimized and the potential values of catalysts for oxygen evolution decrease and the sample with 70 mol% IrO2 has the same as the rate for the OER of the sample with 50 mol% IrO2. They are similar to the results of the CV curves shown above. However, the corrosion currents data of oxide catalysts observed in table 1 confirmed that the highest stablity is for pure IrO2 electrocatalyst compared to the pure RuO2. When more RuO2 is added, the properties of the mixtures are shifted towards smaller stablity.
Figure 5. Polarisatiom curves of IrxRu1−xO2 electrocatalytic powers in solution of 0.5 M H2SO4; scanning potential velocity 1 mVs−1.
Download figure:
Standard image High-resolution imageTable 1. Electrochemical parameters for IrxRu1−xO2 electrocatalytic power.
| IrxRu1−xO2 | EC (mV;SCE) | EOER (mV;SCE) | IC (mV) |
|---|---|---|---|
| RuO2 | 470 | 1.197 | 0.241 |
| Ir0.5Ru0.5O2 | 474 | 1.206 | 0.223 |
| Ir0.7Ru0.3O2 | 546 | 1.204 | 0.244 |
| IrO2 | 306 | 1.244 | 0.172 |
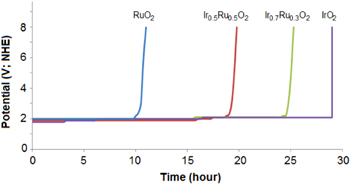
Figure 6 is the galvanostatic results in solution of 0.5 M H2SO4 at current density 200 mA cm−2 (figure 5). RuO2 as an anodic electrocatalyst is unstable with the potential rising to 8 VSCE after 8 h of lifetime. Ir0.5Ru0.5O2 and Ir0.7Ru0.3O2 show higher stability than RuO2 and the lifetimes approach 18.5 h and 24 h, respectively. IrO2 is the best stable anodic electrocatalyst with lifetime of 29 h. Therefore, with the addition of iridium content, the electrocatalytic stability of IrxRu1−xO2 compounds is improved considerably at high potentials in the acidic electrolyte.
Figure 6. Galvanostatic diagrams of IrxRu1−xO2 electrocatalytic powers in solution of 0.5 M H2SO4 at current density 200 mA cm−2.
Download figure:
Standard image High-resolution image4. Conclusion
The IrxRu1−xO2 oxide powder catalysts were synthesized by hydrolysis method. A single rutile-like phase was observed in the XRD pattern and the size measured by SEM is smaller than 50 nm. The research suggested that the IrxRu1−xO2 oxide powder mixtures is more active than IrO2 and more stable than RuO2. With the presence of ruthenium in 30 mol%, the activity and stability of the oxide powder can be improved considerably.