Abstract
Manganese oxides are important materials with a variety of applications in different fields such as chemical sensing devices, magnetic devices, field-emission devices, catalysis, ion-sieves, rechargeable batteries, hydrogen storage media and microelectronics. To open up new applications of manganese oxides, novel morphologies or nanostructures are required to be developed. Via sol—gel and anodic electrodeposition methods, M (Co, Fe) doped manganese oxides were prepared. On the other hand, nanostructured (nanoparticles, nanorods and hollow nanotubes) manganese oxides were synthesized via a process including a chemical reaction with carbon nanotubes (CNTs) templates followed by heat treatment. Scanning electron microscopy (SEM), transmission electron microscopy (TEM), cyclic voltammetry (CV) and impedance spectroscopy (EIS) were used for characterization of the prepared materials. The influence of chemical reaction conditions, heat treatment and template present on the morphology, structure, chemical and electrochemical properties of the prepared materials were investigated. Chronopotentiometry (CP) and CV results show high specific capacitance of 186.2 to 298.4 F g−1 and the charge/discharge stability of the prepared materials and the ideal pseudocapacitive behaviors were observed. These results give an opening and promising application of these materials in advanced energy storage applications.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 3.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Manganese oxides (MnO2) are natural components of soils, aquifers and sediments, and are known to be strong adsorbents of metal ions. Synthetic manganese oxides with micro- and nano-structures are still attracting attention due to their wide applications in different fields, such as catalysis, ion-sieves, rechargeable batteries, chemical sensing devices, magnetic devices, field-emission devices, hydrogen storage media and microelectronics [1–5]. Micro-, meso- and nano-porous manganese oxides, as well as layered claylike manganese oxide nanomaterials are prepared via various routes. There are many reports in the literature regarding synthetic processes that include either oxidation of Mn(II) in basic solution [6, 7], or oxidation by MnO4− [7], O2, K2S2O8, and H2O2 [8] or by reduction of MnO4− using different routes [9]. The reduction of KMnO4 in dilute aqueous H2SO4 produces manganese oxide nanoparticles with hexagonal layer structure [10]. As far as the synthesis of 1D manganese oxide nanomaterials is concerned, nanowires of α-MnO2 are synthesized using coordination-polymers [11–13], whereas single-crystalline β-MnO2 nanorods are prepared by hydrothermal or electrochemical methods [2, 3, 14, 15].
Electrochemical capacitors (ECs), known as supercapacitors, have attracted great interest as promising energy storage devices due to their high power energy density and long cycle performance in comparison with conventional dielectric capacitors [16, 17]. ECs are being considered for different applications such as power sources for camera flash equipment, lasers, pulsed light generators, and as backup power source for computer memory [18]. They also became of interest in hybrid electric vehicles as an auxiliary power source in combination with battery. ECs can provide the peak power in such hybrid systems when accelerating, and thus the batteries can be optimized primarily for higher energy density and better cycle-life. Due to its satisfactory electrochemical performance, natural abundance and environmental compatibility, manganese oxide (MnO2) and especially, nanostructured manganese oxide is considered one of the most promising electrode materials for supercapacitor applications [14, 15, 19–22].
In this study micro-, meso- and nano-porous structured manganese oxides and also M(Fe, Co) doped MnO2 were prepared via chemical and electrochemical methods. Morphology, physical-chemical properties and electrochemical analysis were investigated to study initially the supercapacitive behavior of prepared materials.
2. Experimental
Amorphous nanocluster manganese oxide (ANCMO) was synthesized via the reaction of diluted 0.1 M solution of potassium permanganate (Sigma-Aldrich), KMnO4, with 6 M solution of hydrochloric acid (Sigma-Aldrich), HCl, at room temperature with ultrasonication. The formed solids are filtered and washed five times with de-ionized water, and then dried in air at room temperature for 24 h. Crystalline manganese oxide nanorods (CMONR) were prepared via heat treatment at 650 °C of unwashed ANCMO (high content of potassium ion) for 5 h. The same reaction with the presence of carbon nanotubes (CNTs) templates forms CNTs coated MnO2 and after heat treatment for 5 h at 400 and 650 °C, amorphous and crystalline manganese oxide hollow nanotubes (AMONTs and CMONTs) were formed.
On the other hand, Co and Fe doped manganese oxides, Mn(M)Ox with M = Co, Fe, were electro-deposited on the graphite plates having 1 cm2 work surface area by anodic deposition. The electrodeposition was carried out in the electrolyte containing 0.15–0.25 M MnSO4, 0.05–0.15 M sulfate salt of Co or Fe, 0.2 M EDTA, pH = 6.5–7.0, T0 = 800 °C, I = 50 mA cm−2. The concentrations of Mn+ (M = Co, Fe) and Mn2+ were varied so that the ratio [Mn+]/[Mn2+] = 0/30; 5/25; 10/20; 15/15 and the total amount of cations was kept constant at 0.3 M ([Mn+] + [Mn2+] = 0.3 M). The oxide electrodes obtained were dried at 100 °C in air for 2 h.
The morphology and chemical compositions of the deposited oxides were examined with scanning electron microscope (SEM), energy dispersive x-ray spectroscopy (EDX), transmission electron microscopy (TEM) and chemical analysis. The average valence of manganese was estimated using iodometric titration and complexometric titration methods. Electrochemical behavior and specific capacitance of the deposited oxides were characterized by cyclic voltammetry (CV) and impedance spectroscopy (EIS) tests in 2 M KCl solution at 25 °C.
3. Results and discussions
SEM and TEM images of the MnO2 nanostructures prepared by the above-mentioned chemical processes are shown in figure 1. As mentioned in the experimental section, ANCMOs are prepared according to the following reaction between KMnO4 and HCl:
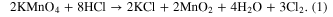
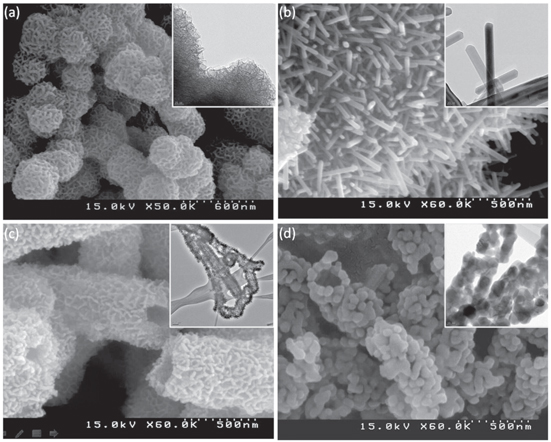
Figure 1. SEM and TEM (inset) images and of nanostructured manganese oxide: (a) ANCMOs, (b) CMONRs, (c) AMONTs and (d) CMONTs.
Download figure:
Standard image High-resolution imageThis figure shows that the nanosclusters are composed of MnO2 worm-like fibers aggregating on the surface of the nanosphere. These fibers appear more tighly packed and slightly smaller in size compared to the control sample (figure 1(a)), whereas the nanorods of the MnO2 which were prepared by heat treatment of ANCMO are well grown and uniform (figure 1(b)). Via the same chemical reaction, with the presence of CNTs templates and after heat treatment, amorphous and crystalline manganese oxide hollow nanotubes (AMONTs and CMONTs) were successfully synthesized (figures 1(c) and (d)). The formation of hollow nanotubes with porous worm-like morphology in amorphous nanotube and granular morphology in crystalline nanotubes was observed clearly.
On the other hand, electrolyzed manganese oxide and M (Co, Fe) doped manganese oxide were synthesized via anodizing process (figure (2)). It can be observed that morphology of manganese oxides has nanoscale fiber-like structure whose radius ranges between 15–20 nm and the surfaces were very porous. When Co2+ and Fe3+ were injected to the electrolyte, surfaces became more porous but porosities of surfaces were different. This is attributed to the difference of electro-deposition rates which are shown in figure 3.
Figure 2. SEM images of doped manganese oxides deposited from different electrolytes (a) [Mn2+] = 0.3 M; (b) [Co2+]/[Mn2+] = 15/15 and (c) [Fe3+]/[Mn2+] = 15/15.
Download figure:
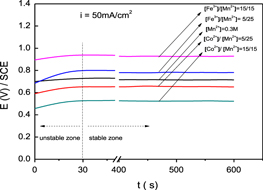
Standard image High-resolution imageFigure 3. Galvanostatic curves of doped manganese oxides deposited from different electrolytes.
Download figure:
Standard image High-resolution imageDuring galvanostatic deposition, the obtained relationship between potential and time was recorded and results are displayed in figure 3. These curves include two zones: (i) unstable zone, which is the first thirty seconds, is characterized by the variation of potentials with time. This zone represents the appearance of manganese oxide on graphite; (ii) stable zone, which begins from the thirtieth second, corresponds to the deposition of manganese oxide on the first dense manganese oxide layer. The value of potential in this zone was almost constant. Despite the deposition at the same density current, the potentials of Fe doped samples were higher than those of Co doped samples. This suggested that the current of the deposition of Fe-doped manganese oxide was smaller than that of pure manganese oxide and much smaller than the one with Co-doping.
The composition of the nanostructured manganese oxides (ANCMOs, CMONRs, AMONTs, and CMONTs) which was determined by EDX and chemical analysis consists of manganese and oxygen only but the compositions of the electrolized manganese oxide and M-doped manganese oxide are different and are summarized in table 1. It can be observed that atomic ratio M/(M + Mn) (M = Co, Fe) increases with increasing [Mn+]/[Mn2+] ratio. This behavior can be explained by the fact that cobalt oxides and ferro oxides were co-deposited with manganese oxides during the process of electrolysis. Oxygen element contents of the obtained manganese oxides are higher than 70%, indicating that hydrated crystal structures were formed during deposition. These can be explained by the co-electrodeposition of Co and Fe with manganese oxides by the following reactions:
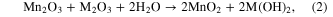
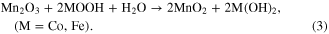
Table 1. Chemical composition of the doped manganese oxidesdeposited from solutions with different [Mn+]/[Mn2+] ratios, (M = Co, Fe).
| Co-doped materials | ||||
|---|---|---|---|---|
| Indeed | [Co2+]/[Mn2+] = 0/30 | [Co2+]/[Mn2+] = 5/25 | [Co2+]/[Mn2+] = 10/20 | [Co2+]/[Mn2+] = 15/15 |
| Co (%at) | — | 0.24 | 0.88 | 0.79 |
| Mn (%at) | 19.18 | 24.55 | 28.54 | 14.98 |
| O (%at) | 80.82 | 75.21 | 70.58 | 84.22 |
| Co/(Co + Mn) | 0.00 | 0.95 | 3.00 | 5.27 |
| Average value of Mn | 3.808 | 3.849 | 3.852 | 3.867 |
| Fe-doped materials | ||||
| Indeed | [Fe3+]/[Mn2+] = 0/30 | [Fe3+]/[Mn2+] = 5/25 | [Fe3+]/[Mn2+] = 10/20 | [Fe3+]/[Mn2+] = 15/15 |
| Fe (% at) | — | 2.32 | 4.48 | 3.82 |
| Mn (% at) | 19.18 | 21.89 | 27.06 | 23.39 |
| O (% at) | 80.82 | 75.79 | 68.46 | 72.79 |
| Fe/(Fe + Mn) | 0.00 | 9.58 | 14.20 | 17.02 |
| Average value of Mn | 3.808 | 3.819 | 3.846 | 3.834 |
The electrochemical performance of the electrolyzed manganese oxide and M(M = Co,Fe)-doped manganese oxide was evaluated by cyclic voltammetry tests in the 2 M KCl electrolyte. The voltammograms measured at various potential scan rates of doped manganese oxides obtained from different electrolytes are shown in figure 4. It can be observed that all the CV curves of electrodes coated with Co- and Fe-doped manganese oxides were symmetric in anodic and cathodic areas. The CV responsive current remained nearly constant during forward and backward scans. It is important to note that the rectangular shapes and mirror image characteristics of these CV curves represent the ideal pseudocapacitive behavior. These results suggested that M(M = Co, Fe)-doped manganese oxides are promising electrode materials for supercapacitive applications. In this figure, although the shapes of CV curves of these materials are similar, Co-doped manganese oxides had a smaller enclosed area than pure manganese oxides. This reflects their degraded charge storage performance. Meanwhile the addition of Fe into materials had more enlarged area which corresponds to their increased charge storage.
Figure 4. Cyclic voltammetry curves of doped manganese oxides. (a) Co-doped materials, (b) Fe-doped materials.
Download figure:
Standard image High-resolution imageHowever, in order to consider the quantifiable influence of doping Co and Fe on this manganese oxide material, variations of the specific capacitance of samples with different potential scan rates and dopant contents were calculated from CV. Results are illustrated in figure 5. At first glance, it is important to see that doping Co increased the specific capacitances for manganese oxide at high sweep rates. After doping Fe the specific capacitances of materials increased at all sweep rates. The maximum specific capacitances achieved at the ratio Fe/(Mn + Fe) = 14.6% (sample obtained from solution [Fe3+]/[Mn2+] = 20/10). This can be explained as the addition of Co and Fe in materials making the average valence of Mn element increase. Moreover, both Co- and Fe-doped manganese oxides had specific capacitances decayed with increasing potential sweep rate. This is attributed to the fact that at high scan rates cations do not keep up with diffusing deeply into layer structure of materials, so the Faradic reactions were prevented.
Figure 5. Dependences of the specific capacitance of materials on the potential scan rates and dopant contents. (a) Co-doped materials and (b) Fe-doped materials.
Download figure:
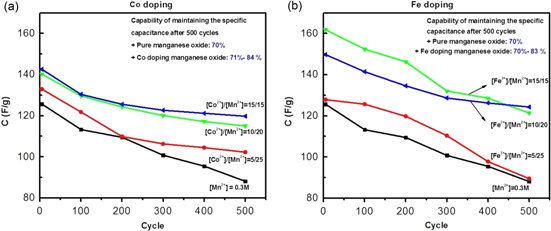
Standard image High-resolution imageElectrochemical stability of the doped oxide electrodes was also evaluated by repeating the CV tests for 500 cycles. Figure 6 shows the variations of the specific capacitance after scanning 500 cycles. As observed, M-doped manganese oxides had the higher charge–discharge stability compared to pure material. The pure manganese oxide retains only 70% of its initial specific capacitance after cycling, while the capacitance retained ratios gain about 84% for Co-doped and 83% for Fe-doped manganese oxides. Higher cyclic stability for Co-doped manganese oxides was also reported by other authors [23, 24]. The decay of specific capacitances can be explained as follows:
- (i)The first is due to corrosion of materials during the active process. If so, doping Co and Fe diminished defects of lattice structure making materials stable and inert.
- (ii)The latter is due to the intercalation or deintercalation of H+ and K+ ion in the bulk of manganese oxides:
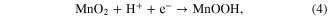
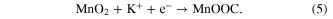
Figure 6. Fading of the specific capacitance of different manganese oxides with CV cycles to clarify those explanations electrochemical impedance spectroscopies were carried out. (a) Co-doped materials, (b) Fe- doped materials.
Download figure:
Standard image High-resolution imageIf the intercalation or deintercalation happens easily and the change of volume of oxides is negligible, the specific capacitances of these oxides decay more slowly after 500 cycles.
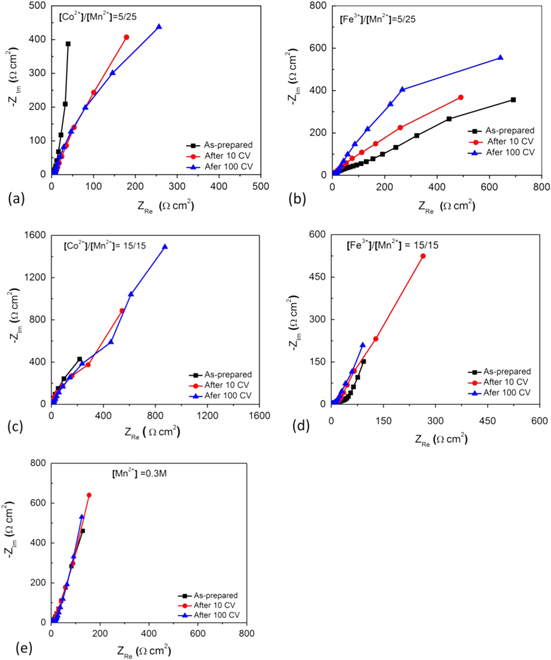
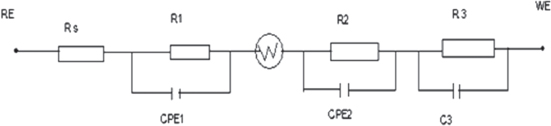
Figures 7 and 8 display Nyquist plots and the equivalent circuit model of manganese oxides electrode. The Nyquist plots of impedance of the manganese oxides electrode consists of an arc and a spike. The arc at higher frequency region should be associated with the charge transfer process at electrode/electrolyte interface [25], and the next part at lower frequency region should be ascribed to the diffusion of cations in the bulk of manganese oxides and the inner contact of the films. The equivalent circuit model shown in figure 8 consists of three parts representing three processes: charge transfer, diffuse and contact. Data obtained by fitting with the circuit model are summarized in table 2, showing that the addition of Co and Fe decreased diffuse resistance, charge transfer resistance and contact resistance of the films. The addition of Co and Fe also increased electric double layer capacitance and capacitor response frequency. These caused the specific capacitance and stability of manganese oxides doped with Co and Fe to increase.
Figure 7. Nyquist of manganese oxides obtained from different electrolytes (a) [Co2+]/[Mn2+] = 5/25, (b) [Fe3+]/[Mn2+] = 5/25, (c) [Co2+]/[Mn2+] = 15/15, (d) [Fe3+]/[Mn2+] = 15/15, (e) Mn2+] = 0.3 M.
Download figure:
Standard image High-resolution imageFigure 8. Equivalent circuit model of the manganese oxide electrodes. Rs: solution resistance; CPE1: constant phase representing double layer; R1: charge transfer resistance; (R2CPE2)W: diffuse impedance of cation into pores of electrode at low frequency; R3: contact resistance between Pt with manganese oxide; C3: capacity between Pt surface and manganese oxide.
Download figure:
Standard image High-resolution imageTable 2. Selected data obtained by fitting the experimental impedance data to the equivalent circuit.
| Oxides obtained from electrolytes | CPE1 (μF) | R1 (Ω cm−2) | R2 (Ω cm−2) | R3 (Ω cm−2) | fφ = 45° (Hz) | |
|---|---|---|---|---|---|---|
| [Mn2+] = 0.3 M | as prepared | 2.201 | 3.76 | 1.62 | 6.27 | 1.22 |
| after 100CV | 3.72 | 4.47 | 4.95 | 9.32 | 0.52 | |
| [Co2+]/[Mn2+] = 5/25 | as prepared | 2.38 | 5.23 | 3.61 | 5.80 | 0.967 |
| after 100CV | 3.81 | 3.43 | 3.23 | 8.65 | 2.11 | |
| [Co2+]/[Mn2+] = 15/15 | as prepared | 12.42 | 5.37 | 2.15 | 4.68 | 4.27 |
| after 100CV | 10.45 | 2.25 | 3.84 | 7.65 | 8.21, 526, 5715 | |
| [Fe3+]/[Mn2+] = 5/25 | as prepared | 10.32 | 3.36 | 4.61 | 5.32 | 53.5, 1600 |
| after 100CV | 7.54 | 4.01 | 2.56 | 7.93 | 0.967 | |
| [Fe3+]/[Mn2+] = 15/15 | as prepared | 9.48 | 4.53 | 2.64 | 6.12 | 0.032, 6760 |
| after 100CV | 8.84 | 3.45 | 3.48 | 8.98 | 10200 | |
The electrochemical performance of the prepared nanostructured manganese oxides are ongoing and will be reported soon but their nanoporous structure, which offers very high specific surface area, promises good application not only in supercapacitors but also in other energy storage systems.
4. Conclusions
Nanostructures of manganese oxides and also M(Fe, Co)-doped manganese oxides were successfully prepared via chemical and electrochemical methods. SEM and TEM analyses confirm the formation of nanocluster, nanorod, nanofiber and hollow nanotubes with porous worm-like morphology in amorphous and granular morphology in crystalline structure. The electrochemical performance of the electrolyzed manganese oxides and also M(Fe, Co)-doped manganese oxides was studied. The highest specific capacitance of 186.2 F g−1 and stability of 84% obtained with the Co-doped manganese dioxide. For Fe- doped manganese dioxide, those parameters were 298.4 F g−1 and 83%, respectively. The Co−, Fe−doped nanostructured manganese dioxide film-coated electrodes showed ideal pseudocapacitive behaviors. The addition of Co and Fe decreased diffuse resistance and transfer resistance of the films. The electric double layer capacitance and capacitor response frequency is also increased. The successful fabrication of amorphous and crystalline nanostructured manganese oxide and also M(Fe, Co)-doped nanostructured manganese oxides give an opening and promising application of these materials in supercapacitor applications.
Acknowledgments
This work is funded by Vietnam's National Foundation for Science and Technology Development (NAFOSTED) (Project Nr. 103.02-2013.76), The World Academy of Sciences (TWAS) Research Grant (No: 14-107RG/CHE/AS_G-Unesco FR:324028603) and Nippon Sheet Glass Foundation for Materials Science and Engineering (NSG Foundation).