Abstract
X-ray diffraction, scanning electron microscopy and transmission electron microscopy showed that TiO2 particles synthesized by a sol–gel procedure exhibited uniform size about 16–20 nm. This nanopowder was deposited on a porous quartz tube (D = 74 mm, L = 418 mm, deposit density ∼16.4 mg cm−2) through an intermediate adhesive polymethylmethacrylate layer to manufacture a photocatalytic filter tube. A polypropylene pre-filter was coated with a nanosilver layer (particle size ∼20 nm) prepared by aqueous molecular solution method. An air cleaner of 250 m3 h−1 capacity equipped with this pre-filter, an electrostatic air filter, 4 photocatalytic filter tubes and 4 UV-A lamps (36 W) presented the high degradation ability for certain volatile organic compounds (VOCs), bacteria and fungi. The VOCs degradation performances of the equipment with respect to divers compounds are different: in a 10 m3 box, 91.6% of butanol was removed within 55 min, 80% of acetone within 100 min, 70.1% of diethyl ether within 120 min and only 43% of benzene was oxidized within 150 min. Over 99% of bacteria and fungi were killed after the air passage through the equipment. For application, it was placed in the intensive care room (volume of 125 m3) of E hospital in Hanoi; 69% of bacteria and 63% of fungi were killed within 6 h.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 3.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Indoor air pollution has received significant attention since the early 1990s because people generally spend more than 80% of their time indoors and the indoor risk from inhalation of pollutants is higher than the outdoors one [1]. Many studies have shown that the level of pollutants in the indoor environment is actually higher than in the outdoor environment [2–4]. According to the World Health Organization (WHO), air pollution causes approximately 3.3 million deaths a year worldwide [5]. The complex hospital environment requires special attention to ensure healthy indoor air quality (IAQ) to protect patients and healthcare workers against hospital-acquired (nosocomial) infections and occupational diseases. Poor hospital IAQ may cause outbreaks of building-related illness such as headaches, fatigue, eye and skin irritations, and other symptoms [6–9]. Consequently, healthy indoor air quality is paramount in medical care facilities including hospitals, nursing homes, health and hospice care centres.
There are many traditional methods for air pollution treatment such as adsorption, separation or using chemical disinfectants, but they have the same weakness: pollutants just move from one place to another without being thoroughly resolved or there is the potential formation of by-products toxic for human health. Lately, the photocatalytic pollution treatment method using nano-TiO2 material has been discovered and is considered as a breakthrough solution [10–13]. Titanium dioxide when being irradiated with ultraviolet (UV) light creates an excited-state of electron (e−) and hole (h+) pairs [14], which are able to react with O2 and water vapor in the atmosphere to produce superoxide ions (O2−) and hydroxyl radicals (OH•). Both O2− and OH• are extremely powerful agents in destroying chemical compounds as well as bacterial cells to form CO2 and H2O [15–20]. For application of this new technology, we synthesized N-doped nano-TiO2 and manufactured a small photocatalytic air cleaner (capacity of 25 m3 h−1) that was equipped with a mechanical pre-filter in order to remove dust particles [21]. However, it is just this pre-filter that keeps and accumulates micro-organisms; after that they can return back into the air. Hence, in the present work, we prevent this drawback by fabricating a big photocatalytic air cleaner (capacity of 250 m3 h−1) and use it to purify the air in a hospital.
Silver has been used as a disinfectant since the times of the ancient Greeks because of its excellent antibacterial activity, low volatility, high stability, unlikelihood of microorganisms' developing resistance and nontoxicity to human cells [22–24]. Its antibacterial activities could be interpreted by the sorption of silver ions onto the negatively charged bacterial cell wall, causing deactivation of cellular enzymes, disruption of membrane permeability, leading to eventual cell lysis and death [25, 26]. Recent studies have shown that silver particles of nano-size caused the strong antibacterial effect because of high specific surface area [27, 28].
In this paper the nano TiO2 was prepared by simple sol–gel method at room temperature and then characterized using x-ray diffraction (XRD), scanning electron microscopy (SEM), transmission electron microscopy (TEM). This prepared nanomaterial was deposited inside a quartz tube which was then installed in an air cleaner. The nano silver synthesized by aqueous molecular solution method was coated onto a pre-filter. The air cleaner was evaluated inside an experimental box by oxidation of some VOC molecules (acetone, diethyl ether, benzene, butanol) and by bacterial degradation (bacteria and fungi). In addition, the air cleaner was placed in the intensive care room (volume of 125 m3) of E hospital in Hanoi for purifying air.
2. Experimental
2.1. TiO2 synthesis and characterization
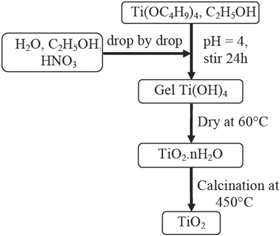
The nano TiO2 was synthesized by a sol–gel process at room temperature (overall procedure shown in figure 1) using tetra-n-butyl orthotitanate Ti(OBu)4 (99% Merck, Germany as a titanium precursor. The mixture containing 20 mL Ti(OBu)4 and 500 mL ethanol (C2H5OH, Merck, Germany) was treated by ultrasonic wave at about 30 min, 20 kHz (called solution A); the mixture containing 60 mL ethanol and 1 mL nitric acid (65% Merck) was treated by ultrasonic wave at about 30 min, 20 kHz (called solution B). Solution B was then drop-wise added in solution A under stirring for 0.5 h and aged for 1 day, the pH of mixture was controlled with HNO3 at a value of 4. Ti(OH)4 gels were dried at 60 °C for 24 h. The obtained xerogels were ground and finally calcined for 3 h at 450 °C to obtain TiO2 nanoparticles.
Figure 1. Overall procedure for preparing nano TiO2 by sol–gel method.
Download figure:
Standard image High-resolution imageMorphology and size of the nanoparticles were examined using SEM (JEOL-JSM 5410, Japan), TEM (JEOL-JEM1010, Japan) and XRD measure using a BRUCKER D8-Advance 5005 diffractometer with Cu-Kα radiation.
2.2. Preparation of anosilver-coated pre-filter and its disincfection property
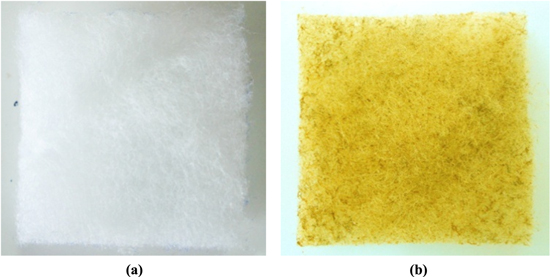
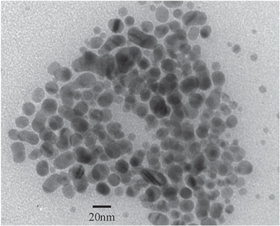
Popypropylen (PP) fabric was immersed in a nanosilver solution (AgNPs) at a concentration of 500 ppm for 2 h, then was naturally dried for 24 h (figure 2). The AgNPs used for coating were synthesized at the Institute of Environmental Technology (IET) by Ngo et al [29] with an average particle size of 20–25 nm (figure 3).
Figure 2. Image of PP filter before (a) and after (b) coating nanosilver.
Download figure:
Standard image High-resolution imageFigure 3. TEM image of a nanosilver sample used for coating.
Download figure:
Standard image High-resolution imageTo determine the disincfection property of nanosilver-coated pre-filter, E. coli strain was employed. The nanosilver pre-filter was cut into 2 × 2 cm2 pieces and immersed in 100 mL of a E. coli solution (106 cfu ml−1 density) for 24 h. Next, 0.1 mL of this solution was extracted and incubated at 37 °C for 24 h then the number of micro-organisms was determined using a count plate method. Microbial counts were expressed as log CFU/m3. A control sample (PP fabric without nanosilver) was also carried out under the same conditions. This experiment was repeated five times.
2.3. Manufacturing air cleaner
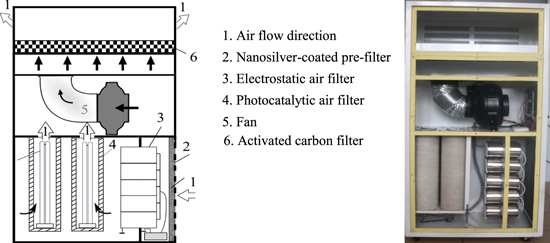
As shown in figure 4, our air cleaner of 250 m3 h−1 capacity is equipped with a nanosilver-coated pre-filter, an electrostatic air filter, 4 photocatalytic filter tubes (PFT) and an activated carbon filter.
Figure 4. Schematic diagram of the air cleaner.
Download figure:
Standard image High-resolution imageThe pre-filter is constituted of two layers: the first is a rude cotton filter to keep the particles larger than 3 μm and the second is a nanosilver PP fabric to keep the particles larger than 0.4 μm.
The electrostatic air filter is made of 10 steel tubes (D = 76 mm, L = 148 mm, thickness = 0.7 mm) and a high-tension power (10 kV). PFT was prepared by deposition of nano TiO2 thin films on a quartz tube fabricated by TIOKRAFT Company (Russia) (D = 74 mm; L = 418 mm, Ssurface = 971.3 cm2) using an intermediate adhesive polymethylmethacrylate (PMMA) layer. Illumination was provided by 4 ultraviolet lamps (UVA, 1.84 mW cm−2 at 360 nm, Phillip) placed in the center of 4 PFT. A 200 W centrifugal fan is used to draw air into the lower portion of the side of the chassis and exhausted out the top of rear of the chassis, as indicated by the airflow directions in figure 4. The actived carbon filter is installed to absorb and eliminate odors.
2.4. Performance of the air cleaner
The air cleaner was placed in a closed box (10 m3 of volume). The air-purification ability was evaluated by oxydation of volatile organic compounds (VOC) such as acetone (C3H6O, Merck), diethyl ether (C4H10O, Merck), benzene (C6H6, Merck) and butanol (C4H10O, Merck). 2.5 mL of each VOC was contained inside the box. The concentrations of VOC at various times was measured by gas sensor TGS2602 (FIGARO). To truly evaluate the removal efficiency of VOC, we used the removal efficiency ηVOC defined by the following equation
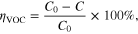
where C0 and C are the initial and final VOC concentration inside the environmental chamber.
The total aerobic bacteria and fungi were selected as model micro-organism to evaluate bacterial degradation ability of the air cleaner. The standard plate count method using plate count agar (PCA) (Merck) was carried out to enumerate the total aerobic bacteria (after incubation at 37 °C for 24 h) and the total fungi after incubation at 37 °C for 48 h). Microbial counts were expressed as log cfu m−3. For each experiment, we determined the quantity of micro-organisms in air before and after the passage of air through the device. Each experiment was repeated three times.
2.5. Hospital air purification by the air cleaner
The air cleaner was placed in the intensive care room (volume of 125 m3) of E hospital in Hanoi. The total aerobic bacteria and fungi were selected as model micro-organisms to evaluate the disinfecting ability of the air cleaner. We determinated the bacteria and fungi density in the room before and after 2, 4 and 6 h of turning on the equipment. At each moment, we took 9 samples at 9 specific positions in the room and each experiment was repeated three times. The Origin software was used for data analysis and graphing.
3. Results and discussion
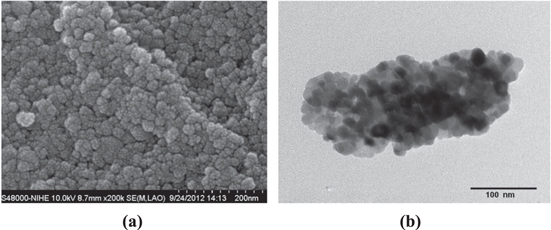
3.1. Characterization of TiO2 synthesized
Figure 5 shows the SEM and TEM images of TiO2 prepared by sol–gel method. It can be seen that the size of the synthesized TiO2 is in the range of nanometers. The particles have spherical form and uniform size about 15–20 nm. The XRD patterns of the sample depicted in figure 6 reveal the strongest peak at 2θ = 25.3° for anatase phase reflections and at 2θ = 27.4° for rutile phase reflections. The average crystallite size of the nano TiO2 is calculated from the XRD data according to Scherrer's equation:
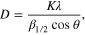
where λ is the wavelength (Å) of characteristic x-ray, β1/2 is half-value with of anatase peak obtained by XRD and θ is 25.3/2 [30]. In this case, the TiO2 crystallites size was estimated to be at 15.84 nm.
Figure 5. SEM (a) and TEM (b) image of TiO2 prepared.
Download figure:
Standard image High-resolution imageFigure 6. XRD patterns of TiO2 prepared by sol–gel method. R: rutile, A: anatase.
Download figure:
Standard image High-resolution imageThe percentage of anatase, A(%), was determined as follows [31]
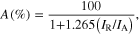
where IR is the intensity of the rutile peak at 2θ = 27.4° and IA is the intensity of the anatase peak at 2θ = 25.3°.
The percentage for the anatase and rutile phases was calculated to be 90.68% and 9.32%, respectively. These results agreed with the data from the SEM and TEM images and the synthesized TiO2 can be used to manufacture the photocatalytic filter of our air cleaner.
3.2. Disinfection property of nanosilver-coated pre-filter
Table 1 demonstrates the disinfection efficiency of nanosilver-coated pre-filter after 24 h of contact time.
Table 1. Disinfection property of pre-filter after 24 h of contact time.
| Sample | Initial E. coli density (cfu ml-1) | Final E. coli density (cfu ml-1) | Efficiency (%) |
|---|---|---|---|
| Ag-PP | 106 | 0 | 100 |
| PP | 106 | 106 | 0 |
The data showed that all bacteria were removed after 24 h of immersing the nanosilver -coated PP fabric (Ag-PP sample) into an E. coli solution of 106 cfu ml−1 density while no bacterium was killed in case of nanosilver-uncoated PP fabric (PP sample), i.e. coating a nanosilver layer can prevent the accumulation of micro-organisms in the pre-filter pores.
3.3. Performance of the air cleaner
3.3.1. VOC oxydation activity
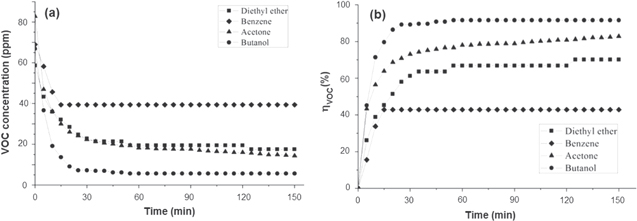
Figure 7 shows the evolution of concentration and removal efficiency of aceton, diethyl ether, butanol and benzene under UV-A light of the air cleaner. It was found that four VOCs could be photocatalytically degraded and the photocatalytic degradation efficiencies of divers VOCs were different. Among these VOCs, butanol was the most easily degradated, namely 91.6% of butanol was removed within 55 min. In contrast, the photo-degradation of benzene in the air was not efficient, reaching only 43% within 150 min. Moreover, the acetone and diethyl ether were more easily oxidized than bezene, namely 80% of acetone was removed within 100 min and 70.1% of diethyl ether within 120 min. This result is consistent with [32, 33] and can be explaind in terms of different adsorption abilities onto the TiO2 of VOCs: butanonol and acetone are more easily adsorbed than diethyl ether and benzene [32], respectively. Furthermore, the carbon chain length of the acetone molecules is the shortest, so it is easly degraded by OH° radicals while the aromatic nucleus of benzene is attacked with difficulty. In addition, some researchers think that benzene could cause catalyst deactivation [34, 35].
Figure 7. Evolution of concentrations (a) and removal efficiency (b) of several VOCs.
Download figure:
Standard image High-resolution image3.3.2. Bacterial degradation activity
Table 2 summarizes the bactericidal and fungicidal efficiencies of the photocatalytic air cleaner when the air passed through the equipment once.
Table 2. Bactericidal efficiency of the photocatalytic air cleaner of 250 m3 h−1 capacity.
| Aerobic bacteria | Fungi | |||
|---|---|---|---|---|
| Time | Density (cfu m−3) | Degradation efficiency (%) | Density (cfu m−3) | Degradation efficiency (%) |
| Before entry into equipment | 488 | 0 | 44 | 0 |
| After exit from equipment | 4 | 99.2 | 0 | 100 |
It was found that the bacterial and fungicidal degradation activity of the photocatalytic air cleaner is very high, more than 99% of bacteria and fungi were degraded after passage of air through the equipment.
3.4. Hospital air purification by the air cleaner
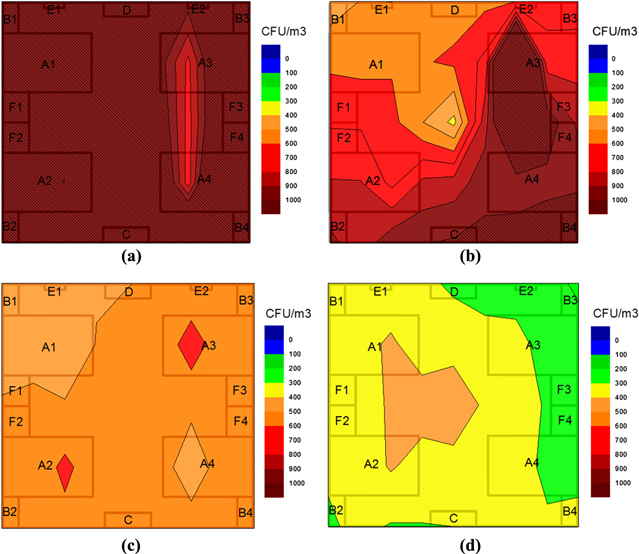
Figure 8 shows the distribution of bacterial density in the intensive care room of E hospital in Hanoi at different moments. It can be seen that the initial bacterial density everywhere in the room was quite high, about 1100 cfu m−3, i.e. the room air was highly contaminated and after running the air cleaner, the density decreased rapidly with time: 52% of bacteria were killed within 4 h on average and 70% within 6 h. In addition, the bacterial density in the area near the equipment decreased more rapidly than the ones in other areas. This is probably due to a weak diffusion of air in the room; the clean air flowing from the equipment needs some time to diffuse toward the far areas. Moreover, during an experiment, the healthcare workers sometimes opened the door and came into the room, so the contaminated outdoor air entered the room.
Figure 8. Distribution of bacterial density in the intensive care room at the moment: before (a) and after 2 h (b), 4 h (c), 6 h (d) turning on the device. A1–A4: patient's beds; B1–B4: patient's sideboards; F1–F4: patient's respiratory devices; C: door; D: position of equipment, E1–E2: air-conditioners.
Download figure:
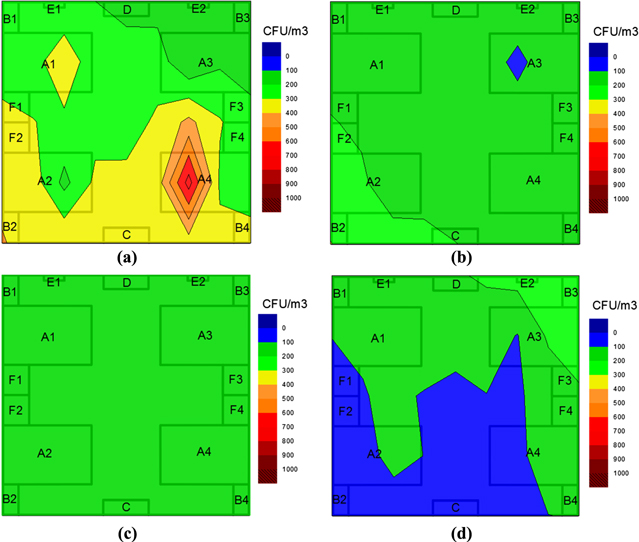
Standard image High-resolution imageFor fungal degradation, the equipment presented a high efficiency, too. Figure 9 shows the distribution of fungi density in the intensive care room of E hospital in Hanoi at different moments. The initial fungi density in the room has the value ranging from 170 cfu m−3 to 400 cfu m−3 and it decreased with time. 51% of bacteria were killed within 4 h on average and 63% within 6 h.
Figure 9. Distribution of fungi density in the intensive care room at the moment: before (a) and after 2 h (b), 4 h (c), 6 h (d) turning the equipment. A1–A4: patients' beds; B1–B4: patients' sideboards; F1–F4: patients' respiratory devices; C: door; D: position of equipment, E1–E2: air-conditioners.
Download figure:
Standard image High-resolution imageConsequently, these results demonstrate that our air cleaner may be used in hospitals to clean indoor air.
4. Conclusion
The TiO2 nanopowder synthesized by a sol–gel procedure using tetra-n-butyl orthotitanate as a titanium precursor consists of spherical particles with a uniform crystallite size about 16–20 nm. The crystal phase presents a mixture of anatase (90.68%) and rutile (9.32%). This powder was deposited on a porous quartz tube (D = 74 mm, L = 418 mm, deposit density ∼16.4 mg cm−2) through an intermediate adhesive PMMA layer to manufacture a photocatalytic filter tube. The nanosilver layer coated on the PP pre-filter could prevent the accumulation of micro-organisms here beacause all bacteria were removed after 24 h of immersing the nanosilver-coated PP fabric (Ag-PP sample) into an E. coli solution of 106 cfu ml−1 density. An air cleaner of 250 m3 h−1 capacity installed with a nanosilver-coated pre-filter, 4 photocatalytic filter and 4 UV-A lamps (36 W) could remove 91.6% of butanol in a 10 m3 box within 55 min, 80% of acetone within 100 min, 70.1% of diethyl ether within 120 min and only 43% of benzene within 150 min. The different photocatalytic degradation efficiencies of equipment for divers VOCs were due to different molecular structures and adsorption abilities onto the TiO2 of VOCs. For bacterial and fungal degradation, over 99% of bacteria and fungi were killed after the air passage through the equipment. When the equipment was placed in the intensive care room (volume of 125 m3) of E hospital in Hanoi, 69% of bacteria and 63% of fungi were killed within 6 h, therefore, the photocatalytic air purification equipment may be used efficiently in hospitals to clean indoor air.
Acknowledgments
This work was supported by the Vietnam–Russia international cooperation project of the Vietnam Academy of Science and Technology (VAST.HTQT.NGA.08/13-14).