Abstract
In this paper we present silver nanoparticles ink synthesis targeting conductive patterns for micro fabricated devices by inkjet printing technology. The well dispersed nanoparticles ink was composed of silver colloid with an average particle diameter less than 10 nm. These nanoparticles were protected by a capping layer of poly(N-vinylpyrrolidone) (PVP) even at silver concentration of 20 wt%. Stable aqueous inks were formulated by using a combination of solvent and co-solvents and under vigorous stirring. Various factors affecting the adhesion between the ink and the substrate were investigated, such as solvent and co-solvent content. The ink containing 20 wt% silver has a viscosity of about 9.5 cP and a surface tension of 32 to 36 mN m−1 at room temperature, meeting inkjet printer requirements. The ink stored under ambient conditions was stable against aggregation for more than one month. Silver nanoparticles patterns have been successfully printed on various substrates.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 3.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
In the past decade, printing techniques such as inkjet printing have been gaining wide interest [1–3] and drop-on-demand inkjet technology developed as a means to achieve an efficient way of point-of-use printing. At present, this non-contact technology has reached such a quality that it allows one to print photographs with unsurpassed quality. The specific advantage of this technology is its ability to print a controlled amount of ink, down to 1 pl, at high frequency, on almost any type of substrate. Thanks to the developments of the technology for consumer purposes, it is now possible to use it for the deposition of materials on any surface. Such developments are progressing at a fast pace and demonstrations have been done in many areas, including the deposition of electrical circuits [4], organic and inorganic transistors [5, 6], coatings for liquid crystal displays (LCDs) [7], biological cells [8], solar cells [9], etc.
The ink is the core of the technology because all final material properties as well as drawbacks are dictated by its chemistry. To name a few, the evaporation, the film homogeneity, the electrical properties, all rely heavily on ink formulation. In this sense, one ink formulation cannot fit all applications. For conductive ink, several studies have reported conductivity of nanoparticles inks at various annealing temperatures [10, 11]. However, the most widely used materials are silver nanoparticles dispersed in an appropriate carrier which allows for proper ink ejection control. This situation also implies that the control on the nanoparticles synthesis is important for the ink development. This study also includes the compatibility of the solvent and co-solvents with the particles as well as the choice of a suitable formulation for the nanoparticles carrier.
In addition to conductivity, the adhesion between ink and substrate is an important parameter in printing applications. Some studies report, for example, new methods for testing the adhesion of nanoparticles inks [12], and the effect of humidity on adhesion [13]. It is also a known fact that contaminants on the printing surface can weaken adhesion, and that some surface treatments such as corona discharge, plasma, or UV-ozone can increase the surface energy and thus improve the wetting of an ink [14]. However, in manufacturing functional devices, excessive wetting may cause problems when printing very fine structures. Actually, decreasing the surface energy has been a better approach when a better control of droplet size is required [15].
In this paper the effect of solvent and co-solvent content on the adhesion between ink and substrate were investigated. Lines printed on different types of substrates were evaluated to determine the effect of substrate types on the adhesion between ink and substrate.
2. Experimental
2.1. Material
All chemicals were purchased and used without further purification: silver nitrate (AgNO3 99%, Merck), ethylene glycol (99%, Merck), polyvinyl pyrrolidone (97%, average molecular weight of 40 000, Merck), acetone (97%, Merck), ethanol (97%, Merck), glycerine (97%, Merck), 2-isopropoxyethanol (99%, Aldrich), ethyl acetate (99%, Sigma-Aldrich), ethyl glycolate (99%, Sigma-Aldrich), ethyl formate (99%, Sigma-Aldrich), sodium dodecyl Sulfate (97%, Merck).
2.2. Preparation of silver nanoparticles and conductive inks
In a typical procedure, an appropriate amount of PVP was dissolved in 20 ml ethylene glycol (EG). AgNO3 was then dissolved into the above solution. Then, an ultrasonic probe was immersed into the mixture solution for three minutes. Upon the start of reaction, the pale yellow solution changed to dark brown, indicating the formation of silver particles.
For the preparation of conductive nanoparticles inks, the synthesized silver nanoparticles were centrifuged and then re-dissolved into organic solvent. Subsequently, the mixture was dispersed with vigorous stirring for approximately 10 min. Finally, the silver nanoparticles inks were printed onto various substrates using a commercial Dimatix printer. A Dimatix materials printer DMP-2800 (Fujifilm Dimatix, USA) is used with 16 nozzle cartridges of 10-picoliter drop volume (DMCLCP-11610).
2.3. Characterization
Synthesized samples were studied by use of UV-visible absorption spectroscopy with a double beam spectrophotometer (Jasco UV–Vis V530) in the wavelength range from 190 to 1100 nm. Transmission electron microscopy (TEM) was used to obtain particle size and shape. Samples for TEM measurements were precipitated by centrifugation at 4000 rpm and redispersed using 1 ml of deionized water to obtain a solution with a high concentration of silver nanoparticles. ImageJ software was used to compute the nanoparticles dimensions. A scanning electron microscope (SEM, JEOL/JSM-6480LV, Japan) was used to analyze the surface morphology of sintered nanoparticles. The viscosity measurements were made with a capillary viscometer (m-VROC) at 25 °C and shear rates of 500 and 20 s−1. The surface tension measurements were done on the CAM 101 from KSV Instruments, equipped with software which can automatically calculate surface tension by analyzing the shape of pendent drop using Laplace–Young equation.
3. Results and discussion
3.1. Silver nanoparticles synthesis
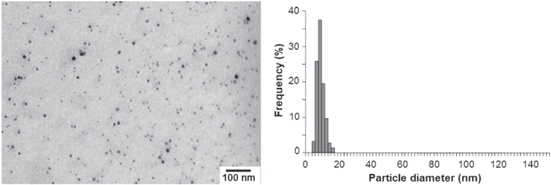
In order to be used as a material in inks, the particle should be small (less than 50 nm in diameter) enough to eliminate clogging in the nozzles of the inkjet printhead during the printing process. In this paper, silver nanoparticles were synthesized by the well-known polyol method which was studied in detail and optimization in our previous work [16]. Figure 1 shows a TEM image and the computed size distribution of colloidal silver particles. The mean diameter of the spherical silver nanoparticles is 10 nm with a narrow distribution ranging from 4 to 16 nm which is suitable for fabrication of conductive inks.
Figure 1. Transmission electron micrographs of Ag nanoparticles and their size distributions.
Download figure:
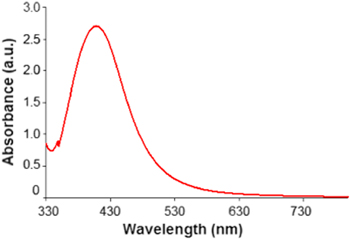
Standard image High-resolution imageFigure 2 shows the UV–Vis spectra of silver colloids of the synthesized solutions. As clearly seen, the optical absorbance appears at a wavelength of 409 nm, which relates to the plasmon resonance of silver nanoparticles [17].
Figure 2. UV-Vis spectra of Ag nanoparticles.
Download figure:
Standard image High-resolution image3.2. Silver ink formulation
The basic requirements for metal-based inks are similar to those of standard inkjet inks, but in addition, they should provide good electrical conductivity of the printed patterns. The ink should demonstrate compatibility with the substrate and good printability and resolution with minimum printer maintenance [18]. In addition, the dispersion of the nanoparticles in ink should be good in order to avoid any aggregation.
Optimization of the silver nanoparticles synthesis (particle size, stabilization) is one of the crucial points in obtaining inks for printing patterns with high electric conductivity. So it requires highly concentrated dispersions of silver nanoparticles loadings (usually 20–40 wt%) [18]. In this paper, the silver nanoparticles concentration reach 9.5 wt% in the as prepared solutions. Moreover, the directly synthesized concentrated dispersions of silver nanoparticles contain high concentration of stabilizing polymer, which are insulators, and therefore are not applicable as inks for conductive printing with low temperature processing.
A conventional method for increasing metal load in dispersions is used, namely separation of silver nanoparticles by centrifugation with acetone and washing the obtained paste with a proper solvent to remove the excess of stabilizing polymer. The obtained nanopowder is then used in the formulation of Ag-based conductive inkjet inks. The first silver ink formulation (I1) prepared here is composed of 20 wt% Ag nanoparticles, 32 wt% ethanol, 32 wt% ethylene glycol and 11.2 wt% 2-isopropoxyethanol, and 4.8 wt% glycerin. It was filtered through a 0.2 μm syringe filter to eliminate large particles.
3.3. Characteristics of silver ink
After the formulation, the ink particles were observed using a TEM. Figures 3(a) and (b) show the TEM image and the computed histogram of particle size distribution of these particles, respectively. The figure clearly shows that the particles are well separated and spherical, the average diameter of silver nanoparticles being about 5 nm with a narrow distribution. The UV–Vis absorption spectra of silver nanoparticles ink is shown in figure 3(c). As is clearly seen, the maximum of the optical absorbance appears at a wavelength of 407 nm.
Figure 3. (a) Transmission electron micrograph of the silver nanoparticles ink (I1), (b) their size distribution and (c) the UV–Vis spectrum of the ink.
Download figure:
Standard image High-resolution imageNow, to obtain a good jetting through the printhead nozzles, the ink should have controlled surface tension as well as viscosity. The silver nanoparticles ink has a measured room temperature viscosity of 12.1 cP and a surface tension of 29.5 mN m−1 at metallic silver concentrations of 20 wt%, indicating that an ink composed of silver nanoparticles has been successfully prepared.
3.4. Substrate materials
Organic substrates have been used in the printable electronics field because of their advantages such as: electrical, chemical and mechanical reliability. To enable the use of additive, direct inkjet manufacturing in combination with nanoparticles materials on organic substrate, the ink-substrate interactions need to be well understood. In this experiment, we used polyethylene terephthalate (PET), glass and silicon as substrates.
The surface quality and smoothness of the selected substrates are important in order to define their surface characteristics. On the other hand, wetting of the ink is also important to achieve high adhesion performance for the inkjet-printed structures. For high adhesion performance, it is crucial to achieve good wetting and low contact angle as close as possible to 0°.
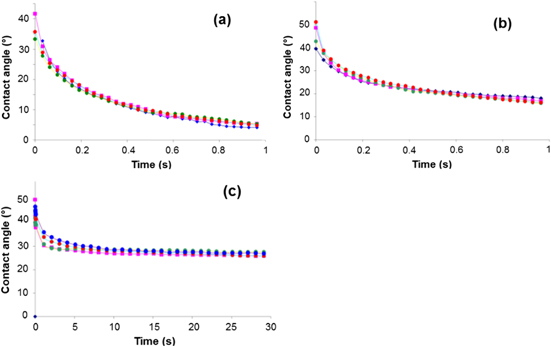
In order to determine the wettability of the ink, its contact angle was measured on all three substrates. PET and glass were cleaned in acetone, rinsed in ethanol and DI water, and then dried with blown nitrogen. Silicon was used out of the wafer box without further treatment, i.e., with its natural oxidized surface. Figure 4 shows the contact angle of the silver nanoparticles ink on the 3 different substrates. Each measurement was reproduced 4 times and always showed a very small variation (less than 1°).
Figure 4. Contact angle of silver ink (I1) on different substrates, (a) silicon, (b) glass and (c) PET.
Download figure:
Standard image High-resolution imageAs can be appreciated, in the case of PET and glass, the contact angle is higher while silicon presents the lowest contact angle. From these values it can be predicted how the suspension will wet the surface, and therefore how the silver nanoparticles will spread. However, we could not find an explanation for the different behavior between these three substrates. Nevertheless, according to [19], such higher contact angle should contribute to only a slightly narrower line width for the ink under study.
3.5. Morphology of printed patterns
Once the ink is confirmed stable and its jetting is also well controlled, an important aspect is the interaction of the droplet with the substrate, i.e. spreading and solvent evaporation. In the case of nanoparticles based inks there is also a subsequent need for consolidation of the deposit through annealing.
In order to ensure good control, the substrate is placed on the printer plate and held in place with vacuum, where it is allowed to stabilize before printing. During the printing process, the distance between the printhead and the substrate was maintained at 1 mm and the substrate heated and maintained at 60 °C. The optimal values of droplet spacing in both X and Y directions were found to be 20 μm. These optimized parameters were used for all the experiments.
We used a 10 pl cartridge supplied by Dimatix. In order to ensure the best conditions for comparing printed lines, only one nozzle of the cartridge was used for all experiments. We assume the droplet volume to be fairly constant over a printed line as found out in literature [20]. Printed lines were one droplet wide for all experiments. The patterns were annealed after printing in a conventional oven over 2 h at 100 °C. This was necessary to stabilize them before transport.
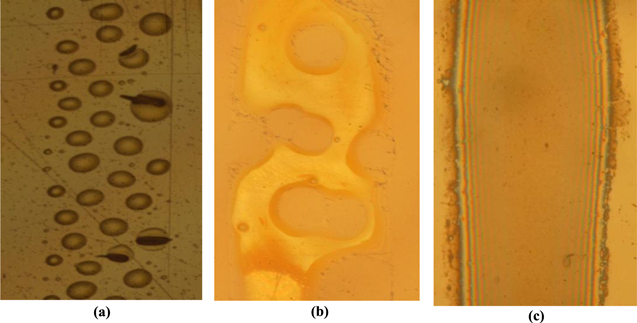
The pictures in figure 5 show printed lines of silver nanoparticles ink on the three substrates. The droplets coalesce into a continuous pattern on silicon substrate (figure 5(c)) while they no longer form a continuous track on PET and glass substrates (figures 5(a) and (b)), instead forming a series of discrete droplets or a wavy pattern. This shows that silver nanoparticles ink presents a proper adhesion on silicon but a poor adhesion on PET and glass.
Figure 5. Printed lines of silver nanoparticles ink (I1) on different substrates, (a) PET; (b) glass and (c) silicon.
Download figure:
Standard image High-resolution imageOne basic aspect in fabricating conductive structures in printable electronics is the adhesion between the ink and the substrate material. Low-cost substrates such as PET (polyethylene terephthalate) could be used in many interesting applications, e.g. in RFIDs, flexible display, organic transistors, or as part of flexible circuit material. In addition, the ink should be processable (annealing, curing) at temperatures below 150 °C, or better 120 °C, to be compatible with such flexible substrates. So it is important to improve the adhesion between silver ink and PET.
There are two ways to improve the adhesion between silver ink and organic substrates. One is surface treatments of substrates such as corona, plasma, or UV-ozone which can increase the surface energy and thus improve the wetting of an ink. The other is controlling the composition of the silver ink. Here the effect of solvent and co-solvent content to the adhesion between ink and substrate were investigated.
The second silver ink formulation (I2) was prepared by dispersing the metal nanoparticles in the form of powder with 20 wt% Ag nanoparticles into organic solvent with proportions as in table 1. It was filtered through a 0.2 μm syringe filter to eliminate large particles. At 25 °C the silver ink has a viscosity of 9.5 cP and a surface tension of 36 mN m−1.
Table 1. Formulation of silver nanoparticles ink (I2).
| H2O (wt%) | Ethanol (wt%) | Ethylen glycol (wt%) | Glycerin (wt%) | 2-isopropoxyethanol (wt%) | Ethyl acetate (wt%) | SDS (wt%) | Ethyl glycolate (wt%) | Ethyl formate (wt%) |
|---|---|---|---|---|---|---|---|---|
| 31 | 15 | 1.5 | 15 | 1.5 | 15.6 | 0.3 | 0.05 | 0.05 |
Figure 6 shows the printed lines of silver ink on PET, glass and silicon substrates. The droplets coalesce into a continuous pattern on all substrate showing a proper adhesion on all three substrates.
Figure 6. Printed lines of silver nanoparticles ink (I2) on different substrates, (a) PET; (b) glass and (c) silicon.
Download figure:
Standard image High-resolution image3.6. Stability of silver nanoparticles ink (I2)
In nanoparticles-based inks, the metallic dispersion in the ink should be stable against aggregation and precipitation in order to prevent nozzle clogging and to obtain ink with reproducible performance. The ink should be stable at least during the process of printing, and in the best case, for printed electronics applications, it should be possible to store the ink for several months as silver is expensive.
To study the stability of silver ink, UV–Vis absorbance spectra, viscosity and TEM images of silver ink were measured at different times, as in figures 7, 8 and 9. The UV–Vis absorbance spectra of silver ink are shown in figure 7. The spectra were measured after 1 day, 15 days, 30 days and 45 days from sample preparation. During the measurement period a blue shift of the absorbance maximum for silver nanoparticles was observed from 410 to 411 and 435 nm, and finally to 535 nm for the surface plasmon resonance of silver nanoparticles. It shows that the ink solution was confirmed to be stable even after 1 month.
Figure 7. The UV–Vis spectra of silver nanoparticle ink at different times.
Download figure:
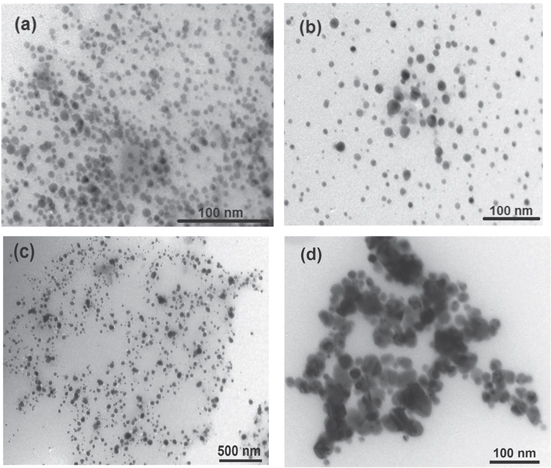
Standard image High-resolution imageMoreover, it was found that there was almost no viscosity change up to 20 days, after which viscosity increased with time from 9.5 cP in day 1 to 10 cP in day 30, as in figure 8. The increase of viscosity from 10 cP in day 30 to 14 cP in day 45 was due to the agglomeration and the formation of larger particle clusters as shown in TEM images below.
Figure 8. Viscosity change of silver ink at different times.
Download figure:
Standard image High-resolution imageFigure 9 illustrates TEM images of silver ink at different times. The mean diameter of the particles is small, as well as with a narrow distribution, and presents no change up to 30 days, after which strong aggregation is observed at 45 days. However, no sedimentation was observed up to 45 days.
Figure 9. Transmission electron micrographs of silver ink at different times. (A) 1 day, (b) 15 days, (c) 30 days and (d) 45 days.
Download figure:
Standard image High-resolution image3.7. Conductive patterns fabrication
In this application, the silver ink is used for printing conductive patterns on PET, glass and silicon substrate, as shown in figure 10. During the printing process, the distance between the printhead and the substrate was maintained at 1 mm and the substrate heated and maintained at 60 °C. The optimal values of droplet spacing in both X and Y directions were found to be 20 μm. These optimized parameters were used for all experiments.
After printing the patterns, it is essential to cure the layer in order to remove excess solvent and increase the conductivity of the silver ink [21]. A curing process also provides the benefit of increasing the adhesion of silver ink tracks on the substrates. Figure 11 shows the difference between heating temperature 100 °C and 200 °C. At lower temperature, the particles do not look connected. When the temperature is increased to 200 °C, the particles start to coalesce, which is visible through the necking between adjacent particles. Curing temperature of 200 °C is then used to sufficiently stabilize the nanoparticles ink.
Figure 10. Photos of printed patterns on various substrates.
Download figure:
Standard image High-resolution imageFigure 11. SEM images of a layer of printed silver nanoparticles ink, after two hours curing at (a) 100 °C and (b) 200 °C.
Download figure:
Standard image High-resolution image4. Conclusion
We have developed a conductive silver nanoparticles-based ink. The well-dispersed stable silver inks were prepared in a mixed solvent to reach suitable viscosity and surface tension. Stored for one month at ambient condition these inks show a good stability. The silver ink was printed onto PET, glass and silicon substrates on which they coalesce into a continuous controlled pattern. We have successfully demonstrated a direct writing of the conductive pattern using silver conductive ink which could serve as an attractive alternative to conventional photolithography for direct patterning conductive tracks at low-cost.
Acknowledgments
The authors highly appreciate the financial support of the Ministry of Sciences and Technology of Vietnam.
Footnotes
- *
Invited talk at the 7th International Workshop on Advanced Materials Science and Nanotechnology IWAMSN2014, 2-6 November, 2014, Ha Long, Vietnam.