Abstract
In this study Fe3O4/CNTs composite with magnetic property was prepared by attaching magnetic nanoparticles (Fe3O4) to carbon nanotubes (CNTs) by hydrothermal method. The obtained Fe3O4/CNTs composite was characterized by Fourier transform infrared (FTIR) spectroscopy, powder x-ray diffraction and transmission electron microscopy. The Fe3O4/CNTs composite was then incorporated into an epoxy coating at concentration of 3 wt%. Corrosion protection of epoxy coating containing Fe3O4/CNTs composite was evaluated by electrochemical impedance spectroscopy and adhesion measurement. The impedance measurements show that Fe3O4/CNTs composite enhanced the corrosion protection of epoxy coating. The corrosion resistance of the carbon steel coated by epoxy coating containing Fe3O4/CNTs composite was significantly higher than that of carbon steel coated by clear epoxy coating and epoxy coating containing CNTs. FE-SEM photographs of fracture surface of coatings showed good dispersion of Fe3O4/CNTs composite in the epoxy matrix.
Export citation and abstract BibTeX RIS

Original content from this work may be used under the terms of the Creative Commons Attribution 3.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Carbon nanotubes (CNTs) have excellent mechanical, electrical, magnetic, optical and thermal properties [1]. They are being studied for some special applications where high-performance materials are required and widely used as a reinforcing material in polymer matrix. In recent years, polymer composites reinforced with CNTs have attracted attention because CNTs enhance mechanical, electrical, thermal and morphological properties of the produced nanocomposite [2–6]. However, the fabrication of CNT-reinforced nanocomposites has two main problems: agglomeration of CNTs and weak interfacial interactions between the CNTs and matrix. To address these problems, many studies have proposed methods to uniformly disperse CNTs and enhance the interfacial interactions in the polymer matrix. The surface modification of CNTs is considered to improve the dispersion of CNTs in a polymer matrix.
Iron oxides such as magnetite, hematite, magnetite and goethite are commonly used as pigments for black, red, brown and yellow colors, respectively. They are widely used as active pigments in organic coatings due to their low cost, easy production and environmental acceptability and also as anticorrosive pigments [7, 8].
In this study, composite nano-Fe3O4/carbon nanotubes (Fe3O4/CNTs) were prepared by hydrothermal method and characterized by Fourier transform infrared (FTIR) spectroscopy, powder x-ray diffraction and transmission electron microscopy. Corrosion protection performance of epoxy coatings containing Fe3O4/CNTs was evaluated by electrochemical impedance spectroscopy.
2. Experimental
The carbon nanotubes (CNTs) used were supplied by Institute of Materials Science, Vietnam Academy of Science and Technology. They were synthesized by catalytic carbon vapor deposition (CCVD) and had a purity of around 95%. The CNTs' diameter ranged from 15 to 90 nm.
HNO3, FeCl3.6 H2O, FeSO4.7 H2O, KOH were purchased from Merck. The epoxy resin was an epoxy bisphenol A, YD-011X75. The hardener was an amine, Sunmide 307D-60. Both compounds were purchased from Kukdo and Air Poroducts and Chemicals.
CNTs were pretreated before the modification by the following procedure: 1 g of CNTs was dispersed in 100 ml of the nitric acid (65 wt%) in a 500 ml round bottom flask equipped with a condenser and the dispersion was refluxed under magnetic stirring for 48 h. The resulting dispersion was diluted in water and filtered. The resulting solid was washed up to neutral pH, and the sample was dried in vacuum at 40 °C overnight.
Magnetite (Fe3O4) was prepared by hydrothermal method (initial molar ratio Fe2+/Fe3+ = 1/1). The precursor was mixed then transferred to a teflon-lined stainless steel bomb, 150 ml capacity, non-stirred autoclave and sealed (the as pre-hydrothermal process). The hydrothermal reaction was conducted in an oven for 7 h and temperatures 150 °C. After reaction, the autoclave was naturally cooled to room temperature, then washed with distilled water and absolute alcohol to remove impurity ions.
Nano-Fe3O4/carbon nanotubes composite (Fe3O4/CNTs) was synthesized using FeCl3.6H2O, FeSO4.7H2O, carbon nanotubes and KOH as starting materials using hydrothermal technique. Before each preparation of nano-Fe3O4/carbon nanotubes composite, the carbon nanotubes were refined in a solution conducted a main compound of HNO3 acid. After that, the purified carbon nanotube was poured into a solution containing a mixture of iron salts and KOH solution. The precursors were mixed by the magnetic stirrer then transferred to a teflon-lined stainless steel bomb, 150 ml capacity, non-stirred autoclave and sealed. The hydrothermal reaction was conducted at 150 °C for 7 h [9]. After reaction, the autoclave was naturally cool to room temperature. The solid was taken out of the Teflon autoclave, and then washed with distilled water and absolute alcohol to remove impurity ions.
The CNTs and Fe3O4/CNTs were incorporated into epoxy resin at concentration of 3 wt%. CNTs and Fe3O4/CNTs were dispersed in epoxy resin by ball mill for 24 h. The liquid paint was applied by spin coating and dried at ambient temperature for 24 h. The dry film thickness was about 20 μm (measured by a Minitest 600 Erichen digital meter).
Fourier transform infrared spectra were recorded with a Nexus 670 Nicolet spectrometer over the range 4000 cm−1–400 cm−1. X-ray diffraction (XRD) patterns were recorded on Siemens D5000 x-ray diffract meter with Cu-Kα radiation. Field emission scanning electron microscope (FE-SEM) observations were done by using a Hitachi S-4800. Transmission electron microscope (TEM) observations were done by using JEM 1010 transmission electron microscopy operating at 80 kV.
For the electrochemical impedance spectroscopy (EIS) measurements, a three-electrode cell was used: the working electrode with an exposed area of 28 cm2, the saturated calomel reference electrode (SCE) and a platinum auxiliary electrode. The corrosive medium was a 0.5 M NaCl solution (reagent grade) in contact with air, quiescent and at ambient temperature. The electrochemical impedance measurements were performed using an Autolab PGStat30 over a frequency range of 100 kHz to 10 mHz with seven points per decade using 30 mV peak-to-peak sinusoidal voltage.
The adhesion strength of the coatings on steel surface was determined by an adhesion tester PosiTest AT according to ASTM D4541.
3. Results and discussion
3.1. Characterization of Fe3O4/CNTs composite
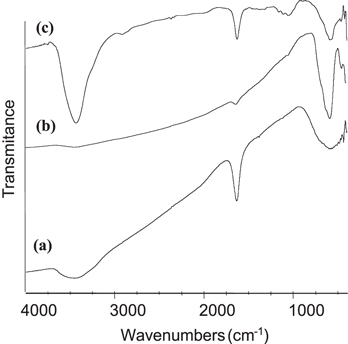
The FTIR spectra of CNTs, Fe3O4 and Fe3O4/CNTs composite are presented in the figure 1. The spectrum of CNTs presents the bands at 3630 cm−1 and 1638 cm−1 characteristic of −OH and C = C group. The spectrum of Fe3O4 has the band characteristic of Fe-O at 578 cm−1 [10]. The FTIR spectrum of Fe3O4/CNTs shows the band at 585 cm−1 corresponding to the vibration of Fe-O link of iron oxide. This result indicates the presence of iron oxide in the Fe3O4/CNTs composite.
Figure 1. IR spectra of CNTs (a), Fe3O4 (b) and Fe3O4/CNTs composite(c).
Download figure:
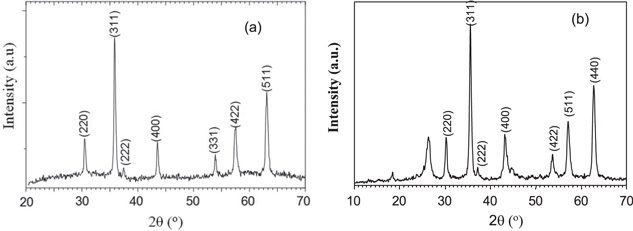
Standard image High-resolution imageThe figure 2 shows the XRD pattern of synthesized Fe3O4 and CNTs composite. In comparison with the Fe3O4 crystal structure in inorganic crystal structure database (ICSD) and other researches all peaks of XRD pattern of synthesized Fe3O4 characterize properly with the standard Fe3O4 [9, 11, 12]. The XRD pattern of Fe3O4/CNTs composite presents characteristic peak of CNTs at 2θ = 26° and all characteristic peaks of Fe3O4. The results indicate that the Fe3O4 nanoparticles are presented in Fe3O4/CNTs composite. Therefore, the Fe3O4/CNTs composite was synthesized.
Figure 2. XRD patterns of synthesized Fe3O4 (a) and Fe3O4/CNTs (b).
Download figure:
Standard image High-resolution imageTEM images of primary CNTs and Fe3O4/CNTs are shown in figure 3. The CNTs have the outer diameter about 30–40 nm. In the image of Fe3O4/CNTs, it is observed that Fe3O4 nanoparticles were adsorbed on the surface of CNTs and the size of nanoparticles is about 10–20 nm.
Figure 3. TEM images of primary CNTs (a) and Fe3O4/CNTs (b).
Download figure:
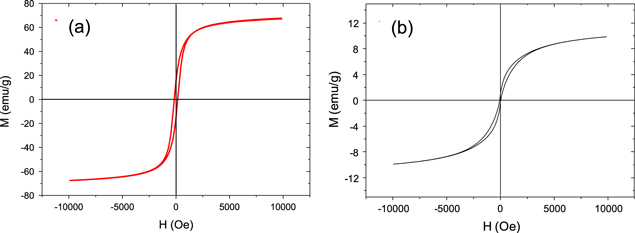
Standard image High-resolution imageThe magnetic properties of Fe3O4 and Fe3O4/CNTs were determined. Figure 4 shows the hysteresis curves of Fe3O4 and Fe3O4/CNTs. The saturation magnetization of Fe3O4 is higher than that of Fe3O4/CNTs composite. These results indicate that Fe3O4/CNTs composites still have magnetic property. Therefore, Fe3O4/CNTs composites can be used in protective coatings to enhance the interaction between the paint and the steel surface.
Figure 4. Magnetic hysteresis curves of Fe3O4 (a) and Fe3O4/CNTs (b).
Download figure:
Standard image High-resolution image3.2. Protection performance of coatings
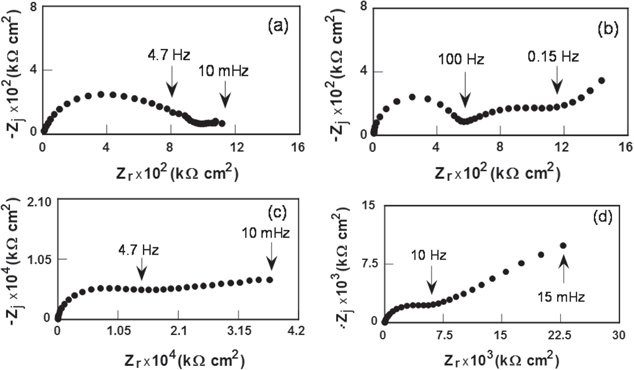
The corrosion protection performance of coatings was evaluated by electrochemical impedance measurements. Figures 5 and 6 present the impedance diagrams obtained after 7 days and 70 days of exposure in NaCl 0.5 M solution for epoxy coating, epoxy coating containing 3 wt% of CNTs, epoxy coating containing 3 wt% of Fe3O4 and epoxy coating containing 3 wt% of Fe3O4/CNTs.
Figure 5. Electrochemical impedance diagrams obtained after 7 days of exposure in the 0.5 M NaCl solution for the carbon steel covered by the epoxy coating (a), epoxy coating containing 3 wt% of CNTs (b), epoxy coating containing 3 wt% of Fe3O4 (c) epoxy coating containing 3 wt% of Fe3O4/CNTs (d).
Download figure:
Standard image High-resolution imageFigure 6. Electrochemical impedance diagrams obtained after 70 days of exposure in the 0.5 M NaCl solution for the carbon steel covered by the epoxy coating (a), epoxy coating containing 3 wt% of CNTs (b), epoxy coating containing 3 wt% of Fe3O4 (c), epoxy coating containing 3 wt% of Fe3O4/CNTs (d).
Download figure:
Standard image High-resolution imageAfter 7 days of immersion, the impedance diagram of epoxy coatings is characterized by one capacitive loop. The impedance diagrams of epoxy coatings containing CNTs, Fe3O4 and Fe3O4/CNTs are characterized by two time constants, but the second time constant is not well defined. Electrochemical impedance diagram of an organic coating was interpreted as follows: the high-frequency (HF) part is related to the organic coating while the low-frequency (LF) part corresponds to the reactions occurring on the metal through defects and pores in the coatings [13, 14]. The electrolyte permeation in the coatings is responsible for stabilizing the corrosion processes in the metallic substrate and for decreasing some of the mechanical and electrical properties of the coating. The resistance values associated with the HF part of epoxy coatings containing CNTs are higher and the resistance values associated with the HF part of epoxy coatings containing Fe3O4 and Fe3O4/CNTs are lower than those obtained for pure epoxy coating.
After 70 days of immersion, the diagrams of coatings have different shapes, pure epoxy coatings have one time constant and the other coatings are characterized by two capacitive loops. The HF loop of pure epoxy coating and epoxy coating containing CNTs decreased significantly, while the HF loop of epoxy coatings containing Fe3O4 and Fe3O4/CNTs decreased slightly.
It was proposed by Kittel et al and the group of Bierwagen that the impedance modulus at low frequencies (such as  or
or  ) measured versus exposure time could serve as an estimation of the corrosion protection of a painted metal [15–18]. Figure 7 plots
) measured versus exposure time could serve as an estimation of the corrosion protection of a painted metal [15–18]. Figure 7 plots  versus immersion time in 0.5 M NaCl solution for the carbon steel covered by pure epoxy and epoxy containing 3 wt% of CNTs epoxy coating containing 3 wt% of Fe3O4 and epoxy coating containing 3 wt% of Fe3O4/CNTs.
versus immersion time in 0.5 M NaCl solution for the carbon steel covered by pure epoxy and epoxy containing 3 wt% of CNTs epoxy coating containing 3 wt% of Fe3O4 and epoxy coating containing 3 wt% of Fe3O4/CNTs.
Figure 7.
 versus immersion time in the 0.5 M NaCl solution for the carbon steel covered by (
versus immersion time in the 0.5 M NaCl solution for the carbon steel covered by ( ) pure epoxy, (◆) epoxy containing 3 wt% of CNTs, (◊) epoxy containing 3 wt% of Fe3O4 and (●) epoxy containing 3 wt% of Fe3O4/CNTs.
) pure epoxy, (◆) epoxy containing 3 wt% of CNTs, (◊) epoxy containing 3 wt% of Fe3O4 and (●) epoxy containing 3 wt% of Fe3O4/CNTs.
Download figure:
Standard image High-resolution imageFor all coatings the impedance modulus decreased rapidly during the first 7 days of immersion. After this exposure time, the modulus at low frequency continues to decrease for the pure epoxy coating and epoxy coating containing CNTs. The impedance modulus at low frequency of epoxy coatings containing Fe3O4 and Fe3O4/CNTs remained relatively stable.
By comparison with the pure epoxy, the impedance modulus of epoxy containing Fe3O4 and Fe3O4/CNT are higher and the impedance modulus of epoxy containing CNTs is lower. The impedance modulus of epoxy coating containing Fe3O4/CNTs is close to those of epoxy coating containing Fe3O4.
The results obtained show that the presence of CNTs in epoxy coating improved the barrier properties of coating. However the effect of Fe3O4/CNTs is much higher than those of primary CNTs. Fe3O4/CNTs enhanced significantly the corrosion resistance of epoxy coatings.
The adhesion of coatings was determined and is presented in the table 1. By comparison with pure epoxy coatings, epoxy-coating-containing CNTs have significantly higher adhesion. The adhesion of coating containing Fe3O4 is higher than the adhesion of epoxy coating, but lower than the adhesion of epoxy coating containing CNTs. The epoxy coatings containing Fe3O4/CNTs has the highest adhesion. The presence of Fe3O4/CNTs improved significantly the adhesion of epoxy coatings. These results are in agreement with the electrochemical impedance measurements.
Table 1. Pull-off strengths for epoxy coating, epoxy coating containing 3 wt% of CNTs, epoxy coating containing 3 wt% of Fe3O4 and epoxy coating containing 3 wt% of Fe3O4/CNTs.
| Sample | Epoxy coating | Epoxy coating + 3 wt% of CNTs | Epoxy coating + 3 wt% Fe3O4 | Epoxy coating + 3 wt% Fe3O4/CNTs |
|---|---|---|---|---|
| Pull-of strengths (MPa) | 0.94 | 2.5 | 1.77 | 3.2 |
FESEM images of the fracture surface of epoxy coating, epoxy coating containing 3 wt% of CNTs, epoxy coating containing 3 wt% of Fe3O4 and epoxy coating containing 3 wt% of Fe3O4/CNTs are shown in figure 8.
Figure 8. FESEM images of a fracture surface of epoxy coating (a) epoxy coating containing 3 wt% of CNTs (b), epoxy coating containing 3 wt% of Fe3O4 (c) and epoxy coating containing 3 wt% of Fe3O4/CNTs (d).
Download figure:
Standard image High-resolution imageIt is observed that in the case of epoxy coating containing 3 wt% of Fe3O4, the Fe3O4 particles are very well dispersed in epoxy. While in epoxy coating containing 3 wt% of CNTs, we can see the agglomeration of CNTs particles. There are the holes between the CNTs particles and epoxy matrix. In epoxy-coating-containing 3 wt% of Fe3O4/CNTs it can be seen that CNTs are well dispersed in epoxy matrix. The good dispersion of Fe3O4/CNTs in epoxy coatings can be explained by the interfacial interaction between Fe3O4/CNTs epoxy through the presence of Fe3O4 on the CNTs surface.
4. Conclusion
Fe3O4/CNTs composite with magnetic property was successfully prepared by hydrothermal method. It was confirmed that the Fe3O4 nanoparticles were adsorbed on the CNTs surface. The corrosion protection of carbon steel by an epoxy coatings containing Fe3O4/CNTs were investigated by electrochemical impedance spectroscopy. It was shown that the corrosion protection of the epoxy coatings was significantly higher in the presence of Fe3O4/CNTs by comparison with the pure epoxy coatings. The Fe3O4/CNTs composite improved significantly the adhesion of epoxy coatings on steel surface. FE-SEM revealed the better dispersion of Fe3O4/CNTs composite in epoxy matrix with CNTs particles.
Acknowledgements
The authors gratefully acknowledge financial support from the National Foundation for Science and Technology Development of Vietnam (NAFOSTED, Project 104.01-2011.01), and the Vietnam Academy of Science and Technology (VAST, Project 03.01/12-13).