Abstract
CuO, Al2O3, Ag2O and La2O3 doped on SnO2 nanoparticles were successfully fabricated by a hydrothermal route at 200 °C for 3 h. The morphology and composition were characterized by field emission scanning electron microscopy (FE-SEM), transmission electron microscopy (TEM) and energy dispersive x-ray analysis (EDX). The results showed that the particle size is 6–8 nm without other impurities. The influence of CuO with a concentration of 0.5–3wt%, which was doped on sol SnO2 5wt% by hydrothermal and vibration technique, was discussed. In addition, the gas sensitivity experiment of samples to 1% LPG in the temperature range of 230–430 °C indicated the improvement in sensitivity and response time. In particular, the SnO2 sol suspension 5wt% doped with CuO 2wt% showed the best sensitivity at 330 °C.
Export citation and abstract BibTeX RIS

Content from this work may be used under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
As an n-type semiconductor, SnO 2 has been widely studied from both scientific and practical viewpoints because of its excellent optical and electrical properties, partly due to its wide band gap (E g =3.6 eV at 300 °C). It is well known that SnO 2 nanoparticles have been fabricated by many methods, such as vacuum evaporation [1], R.F. sputtering [2], spray pyrohydrolysis [3], ball milling [4] and CVD [5]. The study of doping various metal oxides to SnO 2 nanoparticles, especially with sizes in the range of 6–8 nm, has drawn considerable interest for some time. The fact is that most of the doping techniques for SnO 2 sol solution are simple chemical mixing and vibration techniques [6–8]. These are useful if the quantity of additives is small. In contrast, precipitation takes place when doping large amounts of additives to a SnO 2 sol solution.
First used by British geologist Sir Roderick Murchison (1792–1871), the term hydrothermal has been popularized since 1940s by materials scientists. Subsequently, the hydrothermal technique route has found its place in several branches of science and technology, and this has led to the appearance of several related techniques related to the hydrothermal technique, such as hydrothermal synthesis, hydrothermal growth, hydrothermal alteration, hydrothermal treatment and hydrothermal metamorphism [9]. Nowadays, hydrothermal treatment is one of the most important techniques for fabricating nanomaterials. Moreover, the experimental results demonstrated that the hydrothermal treatment route not only made the doping solution more stable but also improved the gas sensing properties of the SnO 2 nanomaterial.
In this paper, the results of doping various metal oxides, like CuO, Ag 2 O, Al 2 O 3 and La 2 O 3, to SnO 2 nanoparticles by the hydrothermal technique have been reported. The sol solution after coating, drying and annealing was characterized by EDX, FE-SEM and TEM, and it was compared with doped solutions derived from a vibration technique. In addition, the possible formation mechanism of fabricating SnO 2 nanoparticles doped with metal oxides was discussed, and the LPG sensing properties of those additives doped on SnO 2 were determined.
2. Experimental
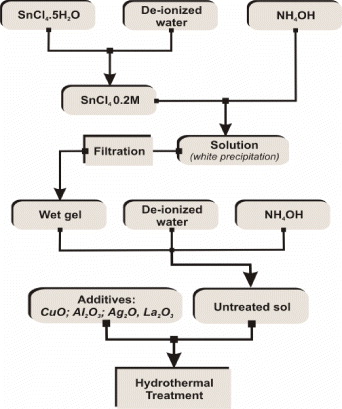
First, SnO 2 nanoparticles were fabricated by a sol-gel method. In a typical synthesis (figure 1), the SnCl 4·5H 2 O crystal was introduced into de-ionized water during stirring for 30 min to receive SnCl 40.2 M solutions. Subsequently, liquid ammonia was dropped slowly into the solution, generating the vigorous reaction as follows:
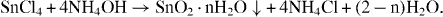
The pH was adjusted to approximately 7 to make sure that the reaction took place completely. After that, the white precipitate was filtered four times with de-ionized water to make stannic acid gel. A transparent suspension containing SnO 2 nanoparticles was produced by dispersing the stannic acid gel back into the distilled water with the support of NH 4 OH. By a traditional technique, we simply mixed untreated sol SnO 2 with Cu(NO 3)2 salt solution by stirring and vibrating. By the hydrothermal technique, the untreated sol was in turn mixed with CuO, Ag 2 O, Al 2 O 3 and La 2 O 3 by stirring for 1 h. Subsequently, the as-mixtures were put into a Teflon-line stainless-steel autoclave (150 mL capacity), sealed and maintained at 180–200 °C for 3 h.
Figure 1 Process of preparation of doped SnO 2 nanoparticles by a hydrothermal technique.
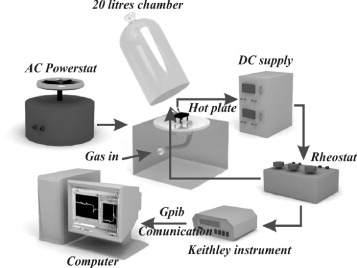
Then, the final solutions were coated on Pt interdigited electrodes by spin-coating (1000 rpm for 5 min). Next, the films were dried, annealed (at 600 °C for 3 h) and transferred for characterization and an LPG sensing test. Gas sensing tests were performed on a static gas test system (figure 2). Finally, the morphology and composition of the samples were characterized by field emission scanning electron microscopy (FESEM; Hitachi S-4800; Institute of materials science, Hanoi, Vietnam), transmission electron microscopy (TEM; JEOL JEM-1010; National Institute of Hygiene and Epidemiology, Hanoi, Vietnam) and energy dispersive x-ray analysis (EDX; FEI-Quanta 200; Institute of Engineering Physics, Hanoi, Vietnam).
Figure 2 Diagram of static gas testing system.
3. Results and discussion
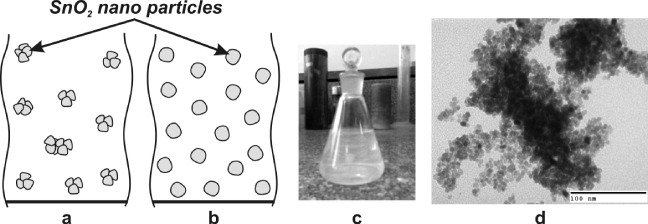
To demonstrate the existence of SnO 2 nanoparticles, two possible models of sol suspension before and after hydrothermal treatment are shown in figures 3(a) and (b), respectively. It was reported previously that the SnO 2 concentrated untreated sol tended to have the colloidal particles agglomerating with each other [10]. In the first model, the grains derived from the wet gel dispersion to dilute water with the support of NH 4 OH exhibited unsteady aggregation among small particles. Therefore, with the increase in SnO 2 weight in the solution, for instance sol 5wt%, 7wt% or 9wt%, particles in the suspended sol aggregated to form bigger grains, which tend to fall according to weight, and this consequence leads to the lack of very uniform grains in the whole solution. In order to create a more uniform solution, the hydrothermal technique was used to provide enough energy with high temperature and pressure conditions to split the group of particles into smaller ones. In addition, it is well known that the particle size and crystal size grow and become more stable with heat after hydrothermal treatment [11]. Thus, this process is useful to favour further poly-condensation and enhance the mechanical properties of the sol suspension. This leads to the formation of a mono-disperse colloid that is highly uniform in shape and colour. Figure 3(c) shows a photograph of a SnO 2 sol suspension 5wt% (5% weight of SnO 2 in suspended solution) treated by the hydrothermal technique after 30 days. Conspicuously, colour and distribution all over the solution are maintained uniformly. The transmission electron micrograph of SnO 2 5wt% treated by the hydrothermal method and annealed at 600 °C for 3 hours is shown in figure 3(d). It can be seen that the particle shape of SnO 2 is sphere-like and the diameters vary across a narrow range (6–8 nm). In addition, the particles are highly uniform and have a good distribution. In spite of the fact that the hydrothermal technique has been studied and popularized since the 1940s, and there were many efforts to illustrate the process inside the hydrothermal condition, it has until now been difficult to explain the formation mechanism. As we know, the hydrothermal treatment involves reactions occurring under the conditions of high temperature and high pressure (>100 °C,>1atm) in aqueous solution in a closed system [12]. Yoshimura's definition (1994) proved that the material has to withstand conditions of high temperature and pressure. Thus, during the hydrothermal treatment, SnO 2 particles or particle groups are exposed to the surrounding pressure of the aqueous solution. The forces not only divide the bunch of particles into smaller ones but distort the undivided particles into a spherical shape. This mechanism may be useful in understanding the doping phenomena using the hydrothermal technique. It should be noted that most related studies are additives doped on SnO 2 nanomaterial using a simple mixing technique [13, 14].
Figure 3 SnO 2 untreated sol model (a), treated sol solution model (b), photograph of SnO 2 sol (c) and SEM image (d) after hydrothermal treatment.
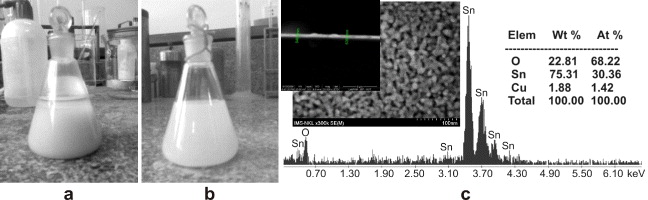
In this study, we introduce the hydrothermal technique as a means to improve the uniformity of material after doping with additives. Figure 4 shows the photographs of SnO 2 5wt% sol suspension doping with 2wt% CuO by traditional and hydrothermal techniques. Conspicuously, the mixture between SnO 2 5wt% sol solution with CuO 2wt% under hydrothermal treatment becomes more uniform and dispersive after 30 days. This phenomenon, in which precipitates fall to the bottom of solution, may depend mainly on the nature of the doping technique. In hydrothermal treatment, the whole solution seems to be uniform without any precipitates, compared with the sample derived from the vibration technique. There are two proposed possible models of doping or mixing additives, which relate to the traditional and hydrothermal techniques. The particle distribution in the first model can be seen as interposition of additives among SnO 2 nanoparticles when doping by a simple technique, like mixing or vibrating. This may cause irregular distribution among SnO 2 particles and additives. This reduces the influence of additives on SnO 2 gas sensing properties because these properties depend strongly on the effective area of material film that is exposed directly to the target gas [15]. Moreover, the functionality of additives not only increases the concentration of reactant at the surface but also decreases the activation energy for the reaction, or both [15]. Hence, beyond the type of additives, good dispersion of additives plays an important role in the improvement of SnO 2 sensing properties. This is different from the vibration technique, in that the hydrothermal technique provides high temperature and pressure, making the residence of metal or metal oxide additives on the surface of the SnO 2 particles in the form of dispersed clusters. This occurrence may be understood as the compression of pressure under hydrothermal conditions. Obviously, uniform dispersion of additives on SnO 2 material may promise a gigantic improvement in SnO 2 gas sensing properties.
Figure 4 Samples of 2wt% CuO/SnO 2 by vibration (a), and hydrothermal treatment (b), FE-SEM and EDX result of 2wt% CuO/SnO 2 derived from hydrothermal treatment (c).
The CuO 2wt% doped, SnO 2 5wt% derived from hydrothermal treatment was then analysed by EDX, as shown in figure 4(c). This result indicates the accurate calculation between SnO 2 and CuO. In addition, it shows that there are no other impurities in the sample. FE-SEM images of 2wt% CuO/SnO 2 in figure 4(c) clearly shows the uniform film (nearly 540 nm thick), which was achieved by spin coating.
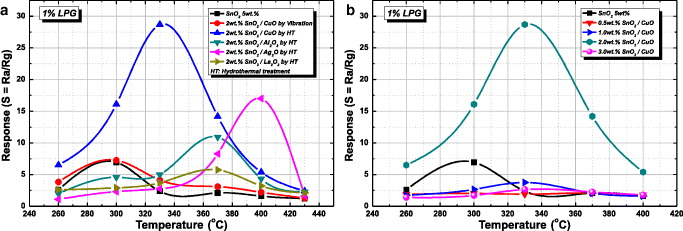
In turn, the Al 2 O 3, Ag 2 O and La 2 O 3 2wt% doped SnO 2 nanoparticles were carried out and coated on Pt electrodes in the same conditions as the CuO doped, SnO 2 process. The influences of additives on the LPG response are presented in figure 5. The most striking feature is that SnO 2 doped with CuO by hydrothermal treatment exhibits the strongest response at 330 °C (approximately 30). Subsequently, there is a downward trend in the responses of Ag 2 O, Al 2 O 3, CuO (doped by vibration) and La 2 O 3 doped SnO 2. It should be pointed out that pure SnO 2 and SnO 2 doped with CuO, Al 2 O 3, Ag 2 O and La 2 O 3 samples have different optimum operating temperatures at 300, 330, 370 and 400 °C, respectively. This result also proves that doping by the hydrothermal technique aids the gas sensing properties of SnO 2 better than the other method. Moreover, 0.5wt–3wt% doped SnO 2 5wt% sol solution were used. Figure 5(b) shows the best response to LPG of CuO 2wt% doped SnO 2 sol suspension in the CuO concentration range of 0.5–3wt%.
Figure 5 Response to temperature curve of additives doped on SnO 2 nanoparticles by the hydrothermal technique.
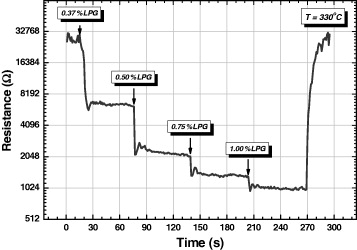
Although there are some shock points (when the film suddenly contacts the LPG) in the characteristic curve of the 2wt% CuO/SnO 2 sample in figure 6, the sample shows a fast response (approximately 15 s) and recovery time (nearly 30 s).
Figure 6 The response–recovery time curve of the 2wt% CuO/SnO 2 sample.
4. Conclusions
In summary, uniform SnO 2 sol suspensions doped with CuO, Ag 2 O, Al 2 O 3 and La 2 O 3 were successfully fabricated by a hydrothermal route with an average particle size of 6 nm. Also, in the possible formation mechanism of SnO 2 nanoparticles under hydrothermal conditions, the hydrothermal technique is potentially advantageous for mass doping. In addition, the LPG sensing experiment indicated that the CuO 2wt% doped SnO 2 5wt% sol suspension in the hydrothermal treatment exhibited better gas sensing properties in comparison with pure SnO 2 as well as SnO 2 doping by vibration.
Acknowledgment
This work was supported by the application-oriented basic research program 2009–2012, code 06/2009/HD-DTDL.