Abstract
In this paper, we report the results of the surface oxidation of platinum (Pt) microwires in aqueous sulfuric acid (H2SO4) solutions by using a cyclic voltammetry technique. The Pt microwire chips were scanned and applied with voltage potentials ranging from 0 to 1.4 V in the H2SO4 solution with concentrations from 0.0003 to 0.0018 M to find out the optimized concentration of sulfuric acid for the oxidation process. The cyclic voltammetry (CV) measurements show the oxidation peak at a potential range from 1.1 to 1.2 V. This is the peak of the interfacial place exchange of chemisorbed O (Ochem) and surface Pt atoms, resulting in the formation of a quasi-3D surface PtO lattice comprising Pt2+ and O2−. The oxidized surface Pt microwires were then functionalized with a 3-aminopropyl triethoxy silane (APTES) and glucose oxidase (GOD) was immobilized onto the functionalized chips for further application in glucose detection. By using this process, Pt microwires have been used for the successful detection of glucose in solution with concentrations in the range of 4–20 mM.
Export citation and abstract BibTeX RIS

Content from this work may be used under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Solid electrodes based on metal materials, especially platinum, are currently in widespread use in electroanalysis, primarily because of its broad potential window, low background current and rich surface chemistry, and suitability for various sensing and detection applications. Surface properties are especially of concern because the interaction of any metal electrode with its environment mainly occurs at the surface, and also because of the dependence of the response on the surface state of the electrode. Many analytical applications, such as electron transfer reaction, preferential accumulation, or selective membrane permeation, can benefit from chemically modifed electrodes [1–3]. Other important applications including electrochromic display devices, controlled release of drugs, electrosynthesis, corrosion protection, etc [4–8] can also benefit from the rational design of electrode surfaces. Accordingly, deliberate modification of electrode surfaces can thus meet the needs of many electroanalytical problems [9, 10], and may form the basis for new analytical applications [11–13] and different sensing devices [14, 15].
One of the most important applications of a platinum (Pt) microwire electrode is glucose detection. To obtain a sensitive and realizable Pt-based glucose biosensor, one of the key steps is enzyme immobilization on the Pt surface for subsequence catalyst oxidation of glucose into sensible products. Up to now, various modification techniques have been applied in surface activation to immobilize the enzyme onto the Pt microwire electrode surface such as physical adsorption [16], entrapment [17], covalent binding [18], cross linking, etc. Among these surface modification techniques, covalent binding produces high surface loading and low enzyme loss due to desorption. Typically, covalent immobilization of enzymes onto metal surfaces involves the functionalization of metal oxide using silane coupling reactions. It is known that silane coupling reactions are capable of providing high surface loading, and the oxide interface typically shows good thermal stability. Because Pt is a noble metal, it is not coated with a thin layer of native oxide. Therefore, in order to functionalize with silane reactants, Pt microwires were oxidized in aqueous sulfuric acid (H 2 SO 4) solutions using a cyclic voltammetry technique [19, 20] in our work. The proposed oxide-formation mechanism at the Pt electrodes is shown in figure 1 and the equations below [19]:
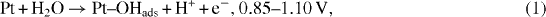
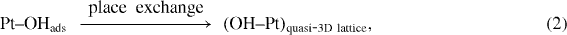
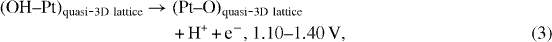
where equation (
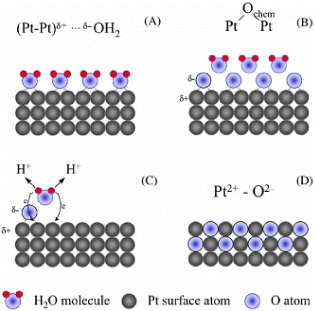
Figure 1 Visual representation of the platinum-oxide growth mechanism: (A) interaction of H 2 O molecules with the Pt electrode; (B) discharge of a half monolayer (ML) of H 2 O molecules and formation of 0.5 ML of chemisorbed oxygen (O chem ); (C) discharge of the second half-monolayer of H 2 O molecules; the process is accompanied by the development of repulsive interactions between (Pt–Pt)δ+–Ochem δ− surface species that stimulate an interfacial place exchange of O chem and Pt surface atoms and (D) quasi-3D surface PtO lattice comprising Pt 2+ and O 2− moieties that was formed through the place-exchange process.
2. Experimental
2.1. Chemicals
D-Glucose, Glucose oxidase (GOx, EC 1.1.3.4, 172 000 units g −1 from Aspergillus niger) were purchased from Sigma Aldrich; cysteamine, carbodiimide and other chemicals were from Sigma Aldrich and Merck. All the chemicals were used as received without further purification. Phosphate buffer saline (PBS) solutions were obtained by mixing 0.05 M KCl with 0.05 M K 2 HPO 4/KH 2 PO 4. Fresh glucose solution was prepared in 0.5 M PBS at pH 7.0 and left at 4 °C overnight to allow the equilibration of the anomers. All aqueous solutions were prepared with deionized water. Electrochemical measurements were taken with a conventional three-electrode cell system. Pt microwire chips were fabricated by a Deposition and Etching under Angles (DEA) technique described elsewhere [21].
2.2. Electrode modification
Chip cleaning: Pt microwire chips were immersed in dicholoromethane, propanol, acetone and deionized water (DI) for 5 min, respectively. Then the samples were dried with blown nitrogen and cleaned by using oxygen plasma (power of 250 W for 6–7 min).
Surface oxidation: After the above cleaning procedures, Pt microwire contained chips were scanned in an aqueous H 2 SO 4 solution using the CV technique with H 2 SO 4 concentrations ranging from 0.3 to 1.8 mM and potential range from 0 to 1.4 V.
Enzyme immobilization: Pt microwire chips were immersed in a solution of 2% of 3-aminopropyl triethoxy silane/ethanol 98% for 6 h, then rinsed with ethanol 3 times. The chip was finally dipped in glucose oxidase solution, which is a mixed solution of carbodiimide with acetate buffer (pH=5).
Enzyme immobilization using a self assembly monolayer (SAM) technique: In order to form a SAM on a Pt electrode, the Pt microwire chip was sonicated in acetone and purified water successively to clean the surface. The cleanness of the surface was verified by using CV in sulfuric acid solution (figure 2). The electrode was then immersed in an ethanol solution of cysteamin HSCH 2 CH 2 NH 2 (Cys) for 10 h at room temperature. A crucial requirement for obtaining a stable SAM is that the Pt surface must be in the reduced state, and this was achieved by keeping the potential of the electrode at −0.3 V versus Ag/AgCl electrode for about 3 min in 0.05 M sulfuric acid solution before performing a subsequence step of thiol adsorption. The C–V characteristics of the samples after the thiol adsorption step is shown in figure 3, and it can be seen that the peaks of hydrogen adsorption are completely depressed. Subsequently, the SAM coated Pt electrode was immersed in a solution of glucose oxidase/acetate buffer (pH 5) 1:2 for 2 h at 4 °C, and then rinsed with DI water.
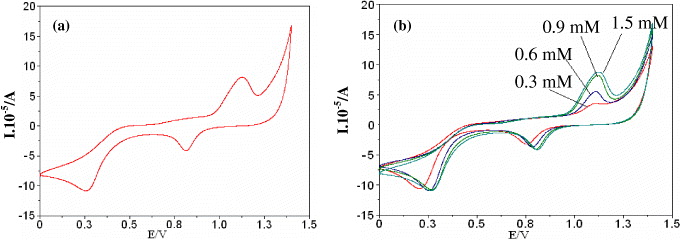
Figure 2 Cyclic voltammetry (I vs E) curves for a Pt microwire electrode (a) 1.5 mM aqueous H 2 SO 4 solution, recorded at s=50 mV s −1 and T=298 K, and (b) in different concentrations of H 2 SO 4 solution.
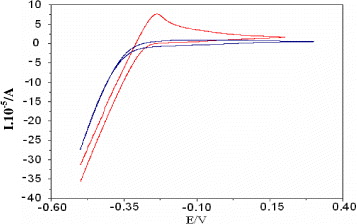
Figure 3 CV curves of Pt electrodes in 0.05 M H 2 SO 4 solution.
2.3. Electrochemical measurement
A conventional three-electrode cell, consisting of a Pt microwire modified electrode as the working electrode, Ag/AgCl, 3 M KCl as reference, and a platinum foil as counter electrode, was connected to the electrochemical system. Autolab PGSTAT30 (Ecochemie, The Netherlands) equipped with EG&G FRA1025 was used for electrochemical measurements. Data were acquired using GPES 4.9 software. The Pt-thiol and Pt-thiol-GOx SAMs electrodes were electrochemically characterized in the presence of H 2 SO 4 solution. Quantitative determination of glucose was carried out using the CV technique.
3. Results and discussion
Pt surface oxidation: The CV transient (I vs E) of a Pt microwire electrode in 1.5 mM H 2 SO 4 solution recorded at s=50 mV s -1 is shown in figure 2(a), while figure 2(b) shows several CV curves of Pt microwires in the same solution with concentrations of H 2 SO 4 varying from 0.3 to 1.5 mM. Figure 2(a) clearly shows that the potential peak of surface oxidation was at a value of E≈1.10 V, while the peak of oxide reduction was seen at the E≈0.85 V. Based on this result, we can determine an appropriate potential regime, in which the Pt surface oxide is formed. The observed CV transient is rather similar with standard research from the literature. In addition, figure 2(b) also shows an increase of surface oxidation peak when H 2 SO 4 concentrations were increased. Our observed results indicate that the most effective concentration of the H 2 SO 4 used is 1.5 mM because the highest efficiency of surface oxidation was obtained at this concentration.
Enzyme immobilization using self assembly monolayer (SAM): As can be seen in figure 3, there were significant changes in CV curves after Pt electrodes experienced thiol modification, and the peaks of hydrogen adsorption were completely depressed. However, the time dependence of current was not observed when the electrode was cycled in the potential region from -0.3 to +0.3 V.
Glucose detection: After being modified with the APTES/GOx layer, the electrode responded to glucose concentration like a glucose sensor. For example, in figure 4(a) we show the CV characteristics of the modified Pt electrodes at different concentrations of glucose. Moreover, the response current increases with the increasing of glucose concentrations in the potential range from 0.9 to 1 V. This is a crucial observation because it shows the sensitive capability of the enzyme modified Pt electrode with glucose concentration.
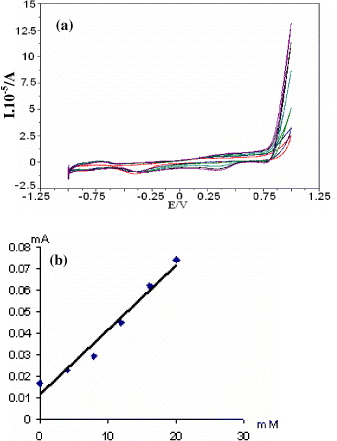
Figure 4 (a) Cyclic voltammetry curves of APTES/GOx modified Pt electrodes in 4–20 mM phosphate buffer (pH 7, 0.5 M) glucose solutions, recorded at scanning rate of 50 mV s −1, (b) A current–concentration dependence of APTES/GOX modified Pt electrodes in 0.5 M phosphate buffer with pH=7.0 at 29 °C.
Based on the observed curves of figure 4(a), we deduce a current–concentration dependence of the modified Pt electrodes, and the result is depicted in figure 4(b). It is very important to state that the observed current–concentration is linear within the experimental concentrations from 4 to 20 mM of glucose solutions, thus allowing the enzyme modified Pt microwire electrodes to be used as glucose sensors for various applications.
Figure 5 shows the Cyclic voltammetry profiles of the Cys/GOx modified Pt electrode in 1–6 mM phosphate buffer (pH 7, 0.5 M) glucose solution. It can be seen that during the increase of glucose concentration from 2 to 4 mM and from 4 to 6 mM the current response decreases. This demonstrates that the modified enzyme is not stable.
Figure 5 Cyclic voltammetry profiles of the Cys/GOx modified Pt electrode in 1–6 mM phosphate buffer (pH=7, 0.5 M) glucose solutions, recorded at a scanning rate of 50 mV s −1: (1) 1 mM; (2) 2 mM and (3) 4–6 mM.
4. Conclusion
Our research proves that the surface of Pt microwire electrodes can be oxidized by using the electrochemical technique in aqueous H 2 SO 4 solutions for subsequent direct and effective immobilization of enzyme onto the electrode surface without using any electron mediator. Furthermore, our work shows that the APTES/GOx modified Pt microwire electrode can be used to detect glucose in solution with concentrations from 4 to 20 mM. Finally, the APTES/GOx modified Pt electrodes obtain better stability and higher sensitivity than that of the Cys/Gox modified Pt electrode.
Acknowledgment
The authors would like to acknowledge the Laboratory for Nanotechnology, Vietnam National University, Ho Chi Minh City for the support of this work.