Abstract
The amphiphilic triblock copolymer of poly(ε-caprolactone)-b-poly(ethylene glycol)-b- poly(ε-caprolactone) (PCL–PEG–PCL) was prepared by ring opening polymerization of PEG and ε-caprolactone in the presence of stannous 2-ethyl hexanoate (Sn(Oct)2) as catalyst. The structure of triblock copolymer was characterized by proton nuclear magnetic resonance (1H NMR), Fourier transform infrared spectroscopy (FT-IR) and differential scanning calorimetry (DSC). The polymeric nanoparticles were prepared in aqueous solution by a co-solvent precipitation technique at room temperature. Nanoparticles were formed from the amphiphilic triblock copolymer, and the effect of organic solvent water-miscibility on the size of nanoparticles was also investigated. Polymeric nanoparticles were measured by dynamic light scattering (DLS), with sizes in the range of 70–90 nm and narrow polydispersity. Additionally, the toxicities of polymeric micelles were evaluated by MTT assay. These results confirmed low toxic polymeric micelles and suggest that the polymeric micelles hold a potential for anticancer drug delivery.
Export citation and abstract BibTeX RIS

Content from this work may be used under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Conventional chemotherapeutic agents have often been associated with limitations due to the drug's poor aqueous solubility, and side effects resulting from their toxicity to healthy tissues. Drugs incorporated into nanosized polymeric micelles are promising nanocarrier systems for drug and gene delivery, because the polymeric micelles have several advantages, such as controlled drug release, enhanced tumor-penetrating ability, reduced side toxicity, increased stability, increased loading capacity and specific-tissue target ability, as well as chemical or physical stimulus sensitivity [1–3].
Poly(ε-caprolactone) (PCL) is a polyester. It has been studied intensively due to its good biodegradability and biocompatibility. PCL can be prepared by ring opening of lactone in the presence of initiation and a catalyst [4]. Nevertheless, PCL is a type of semicrystalline and hydrophobic polymer, which has a melting point around 60 °C. PCL is often at rubbery state at room temperature and has a low glass transition temperature.
Poly(ethylene glycol) (PEG) is a water soluble and nontoxic material with no antigenicity and immunogenicity. Moreover, it is known as one of the promising polymers, which has received approval from the FDA for its internal consumption, which is widely applied in biomedical fields. PEG is considered to be a polymer that can prevent protein absorption and improve the biocompatibility for blood contact compound [5]. In addition, PEG has also been introduced into several polymers, such as PCL, polylactide (PLA), poly(glycolic acid) (PGA) and their copolyesters (PLGA) to increase their biocompatibility and to allow them to be used as drug carriers, which allows PEG to be used for clinical applications. To improve the hydrophilicity and to regulate the biodegradation rate of PCL, random and block copolymers of PCL with PEG are usually prepared. The ester bonding of PCL backbone makes it biodegradable in vivo, and the degradation product, 6-hydroxycaproic acid, can be removed from the body via the citric acid cycle. The poly(ε-caprolactone)-b-poly(ethylene glycol) copolymer displays assured desirable properties for drug delivery applications [6, 7].
It is well known that nanoparticle drug carriers have been utilized for drug targeting issue and biomedical engineering. Therefore, drug delivery systems using nanoparticles are able to increase stability, drug solubility and selective targeting, reduce side effects of anticancer drugs and prolong the blood circulation time [8]. Based on the properties of PCL and PEG, we recently synthesized a copolymer of poly(ε-caprolactone)-b-poly(ethylene glycol)-b-poly(ε-caprolactone), an amphiphilic triblock copolymer, by ring opening polymerization of PEG and ε-caprolactone (CL). The aim of this research was to develop polymeric nanoparticles and to study the effect of organic solvent water miscibility on nanoparticle size. Furthermore, the toxicities of polymeric micelles were also investigated.
2. Experimental
2.1. Materials
Dihydroxyl-terminated PEG with a molecular weight of 4000 g mol −1 was obtained from Sigma-Aldrich Chemical Co. (MO, USA). Before use, this polymer was purified by extraction with ethyl ether, followed by drying through azeotropic distillation in toluene. CL and stannous 2-ethyl hexanoate (stannous octoate, Sn(Oct)2) were purchased from Sigma-Aldrich Chemical Co. and were used as received. All other chemicals were of analytical grade and used without further purification.
2.2. Synthesis of PCL–PEG–PCL
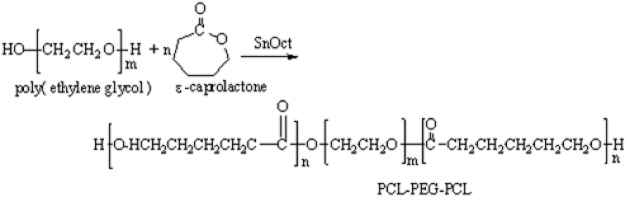
The PCL–PEG–PCL triblock copolymer was synthesized by a ring opening polymerization of PEG and CL in the presence of stannous octoate as catalyst, according to the previous literature [9] with some modifications (scheme 1). A typical procedure was carried out as follows. 4 g of PEG (1.0 mmol) was introduced to a three neck flask and a predetermined volume of CL monomer (22.8 g, 200 mmol) and stannous octoate 0.005 mol% were added to the reaction vessel. The resulting mixture was placed in an oil bath at 130 °C and stirred for 24 h. After that time, the resulting copolymer was cooled to room temperature and dissolved in dichloromethane, and precipitated in an excess amount of cold ether: hexane (1:1, v/v) to remove residual CL monomers and PEG initiators. The triblock copolymer was collected by filtration. Purification of the copolymer was achieved by the dissolution/precipitation method with dichloromethane and ether/hexane (1:1, v/v), respectively, followed by filtration and drying in a vacuum.
Scheme 1 Synthesis of triblock copolymer.
2.3. Characterizations of PCL–PEG–PCL
In order to determine the structure of the triblock copolymer, proton nuclear magnetic resonance (1H NMR) spectra were obtained using Bruker 500 MHz with deuterated chloroform (CDCl 3) as the solvent. Proton assignments were determined from the 1H NMR spectra. Fourier transform infrared (FT-IR) spectra were measured by a FT-IR spectrometer (JASCO Corp, Japan) and the block copolymer-KBr discs were prepared from the polymer-KBr mixtures. In addition, the melting temperatures of the polymers were measured by differential scanning calorimetry (PerkinElmer, USA) under a flow of nitrogen at a scanning rate of 10 °C min −1. Measurements were performed in the range from 5 to 120 °C.
2.4. Preparation of aqueous nanoparticle solutions
The nanoprecipitation method was used to prepare PCL–PEG–PCL nanoparticles [10]. In brief, triblock copolymer PCL–PEG–PCL (5 mg) was dissolved in 2 ml of various organic solvents that are miscible with water. The polymer solutions were added to 10 ml of Millipore water. The resulting solutions were stirred at room temperature for 3 h and transferred to a dialysis bag (MW cut off: 50 000), then dialyzed against Millipore water for 24 h. The solution was filtered through a 0.45 μm syringe filter. The nanoparticles were produced in four solvents: acetone, acetonitrile, N,N-dimethylformamide (DMF) and tetrahydrofuran (THF). The effects of the various solvents were assayed on the overall size of the nanoparticles.
2.5. Determination of particle sizes and polydispersities
The particle size distributions were measured by dynamic light scattering with a Zetasizer 3000 (Malvern instruments, England) at a wavelength of 633 nm and 25 °C, and at a scattering angle of 90° at a concentration of approximately 0.4 mg ml −1 water. The Z average value was recorded as the average of ten measurements.
2.6. MTT assay
Viable cells were assessed using an MTT assay. The L929 cells were seeded at densities designated as 1×105 cells per well into 24-well tissue plates using Dulbecco's modified Eagle medium (DMEM) supplemented with 10% FBS, 100 units ml −1 penicillin and 100 mg l −1 streptomycin. The plates were incubated at 37 °C. After 24 h, the culture medium was replaced with DMEM medium plus 10% FBS, and polymeric nanoparticles of various concentrations were added into the culture medium. The DMEM medium was used as a control. The cells were incubated for 24 h and an MTT assay was performed to evaluate cell viability. Briefly, the culture medium was removed, and the cells were washed with PBS twice. About 160 μl of DMEM medium and 40 μl MTT solution (5 mg ml −1 in PBS) were added to each well, followed by incubation at 37 °C for 4 h to allow formazan formation. The medium and MTT were removed and 200 μl of dimethylsulfoxide was added to dissolve the formazan crystals. After 5 min, the optical density (OD) at 570 nm was determined using a microplate reader. The ratios of OD 570 from each experimental group (n=4) to the OD 570 of the control group were used to assess cell viability.
3. Results and discussions
3.1. Synthesis and characterization of PCL–PEG–PCL
The amphiphilic triblock copolymer was prepared by ring opening polymerization of CL initiated by PEG diol in the presence of Sn(Oct)2 as catalyst, according to scheme 1. The molecular weight of triblock copolymer was calculated from 1 H NMR (M n (PCL-PEG-PCL)=M nPEG +M nPCL ). The molecular weight of the obtained copolymer was 27 810 and similar to the molecular weight calculated from theory (M n =26 820). The typical signals of 1 H NMR spectra of both PEG and PCL were detected when CDCl 3 was used as the solvent, thus confirming that the ring opening polymerization and coupling reaction were carried out. In the 1 H NMR spectra of triblock copolymers dissolved in CDCl 3, the characteristic chemical shifts corresponding to both PCL (1.40, 1.64, 2.28 and 4.04 ppm) and PEG (3.62 ppm) were observed.
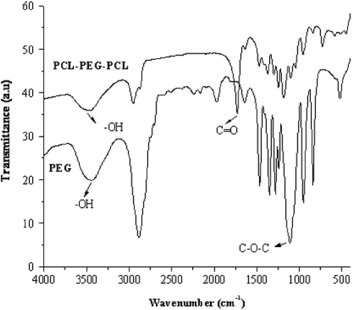
Figure 1 shows the FT-IR spectra of PEG and the PCL–PEG–PCL triblock copolymer. A new strong carbonyl band appears at 1728 cm –1, which could be attributed to the formation of the block copolymer. In addition, the aliphatic CH stretching band of ε-caprolactone is shifted to 2954 cm –1, whereas the absorption band of CH stretching vibration of PEG at 2892 cm –1. The absorption band at 3447 cm –1 is determined to be the terminal hydroxyl groups in the block copolymers. The absorption at 1184–1103 cm –1 is due to C–O stretching. Moreover, a large absorption peak from the C–O–C stretching can be seen at 1184 cm –1 in the FT-IR spectra of the triblock copolymer.
Figure 1 FT-IR spectrum of triblock copolymers.
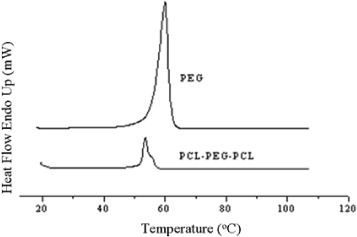
DSC results are shown in figure 2. There are two kinds of thermal curves of PEG–PCL copolymers. The first one is the thermal curve showing a monomodal melting peak, and the second one is the thermal curve resulted in a bimodal melting peak. The higher temperature endotherm can be attributed to the melting of the PCL crystal phase. The low temperature peaks correspond to the melting of the PEG crystal phase. The bimodal melting peaks of the copolymer system were reported by Bogdanov et al [11]. The bimodal melting peak observed for PEG is again most likely due to the existence of folded-chain lamellae with different fold numbers. In our DSC results for the PCL–PEG–PCL triblock copolymer, the melting endotherm showed monomodal melting peaks at 53.6 °C. These results may be attributed to the melting endotherm of the PEG block, which is shifted and less pronounced in the low molecular weight ranges.
Figure 2 Differential scanning calorimetry diagram of triblock copolymers.
3.2. Effect of solvents to control nanoparticle size
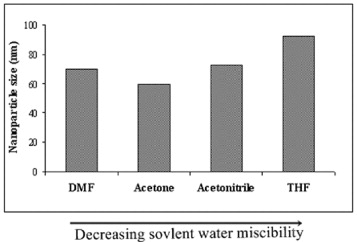
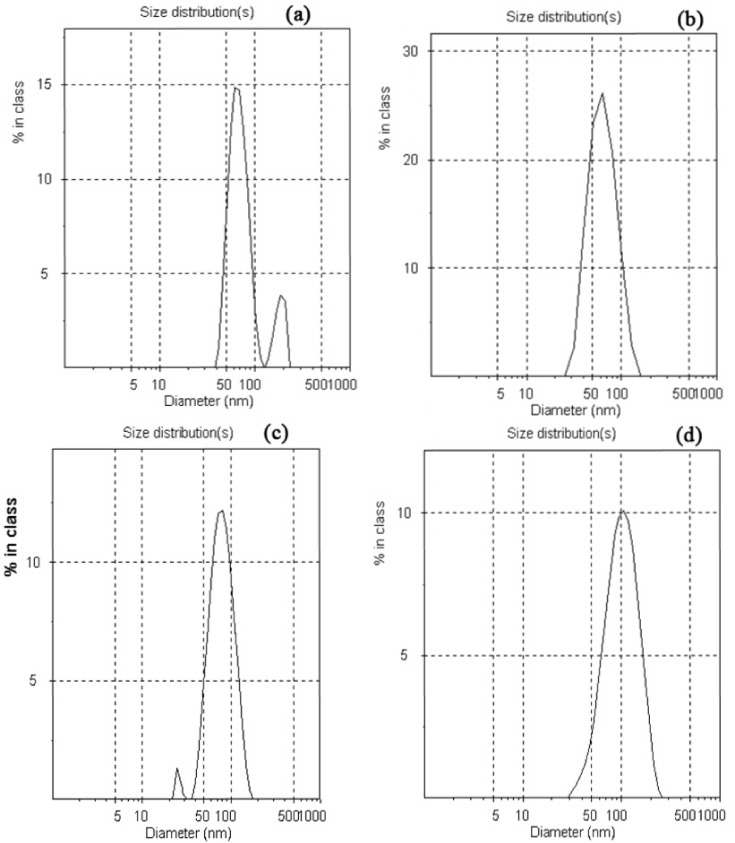
The amphiphilic PCL–PEG–PCL with a hydrophilic PEG block and hydrophobic PCL blocks can self-assemble to form nanoparticles in an aqueous environment. The formation of the hydrophobic PCL inner core and the PEG outer core in an aqueous solution has been reported elsewhere [12]. To investigate the size of nanoparticles of the triblock copolymer in an aqueous milieu, DLS was employed to evaluate the size and size distribution of the obtained nanoparticles. Various water-miscible solvents, such as acetonitrile, acetone, DMF and THF, were used for the preparation of core-shell type nanoparticles of the triblock copolymer. The nanoparticles sizes of the triblock copolymer were 70, 59.4, 72.6 and 92.2 nm for DMF, acetone, acetonitrile and THF, respectively.
As shown in figure 3, the size of the triblock copolymer and the organic solvent water miscibility are generally correlated: a decrease in water miscibility (as indicated by the arrow) led to an increase in the average nanoparticle size. Among these, THF and acetone resulted in the largest and smallest particle size with a narrow size distribution, and maintained a stable nanoparticle solution after the dialysis procedure. Two populations of nanoparticles were observed with DMF and acetonitrile, with a smaller one with DMF with sizes ranging from 20 to 40 nm and a larger one from 45 to 120 nm. In contrast, with acetonitrile, a larger one with sizes in the range of 40–120 nm and a smaller one from 120 to 180 nm (figure 4) were achieved. Similar changes were observed for other copolymers [10, 12]. Therefore, the nanoparticles are dynamic systems and are stable upon dilution and organic solvent water miscibility, which is of major interest for intravenous injection.
Figure 3 Effect of solvents on particle size of micelles.
Figure 4 Particle size distribution of PCL–PEG–PCL nanoparticles: (a) acetonitrile, (b) acetone, (c) DMF and (d) THF.
3.3. Cytotoxicity of polymeric nanoparticles toward L929 cells
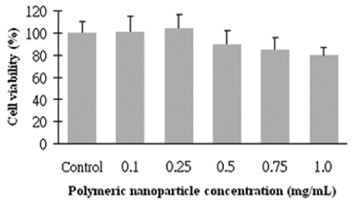
The MTT assay was performed to evaluate the cytotoxicity of polymeric nanoparticles to L929 cells (figure 5). The results of MTT indicated that all concentrations did not significantly affect the cell viability after 24 h incubation with polymeric micelles. Furthermore, the cell viability of concentrations up to 0.25 mg ml −1 was similar to the control group, and 80% of cell viability was observed with a concentration of 1.0 mg ml -1. The cell viability suggests that the polymeric micelle has generally low cytotoxicity to the L929 fibroblast cells with concentrations of up to 1.0 mg ml -1.
Figure 5 Cytotoxicity of polymeric nanoparticles toward L929 cells.
4. Conclusions
We have successfully prepared a biodegradable and biocompatibile triblock copolymer. The structure of the copolymer was confirmed by 1 H NMR, FT-IR and DSC. The nanoparticles of the triblock copolymer are smaller than 100 nm with narrow distribution, in which they may exhibit an enhanced vascular permeability and are a potential carrier of hydrophobic drugs, such as anti-cancer agents [13]. Furthermore, the effect of solvent type on the size of the triblock copolymer was also investigated. This observation was confirmed after evaluating four distinct solvents, and results showed that a decrease in water miscibility led to an increase in the average nanoparticle size. Moreover, the toxicities of polymeric nanoparticles were evaluated by MTT assay. These results confirmed low toxic polymeric nanoparticles and suggested that polymeric nanoparticles have potential for anticancer drug delivery.