Abstract
We report the synthesis of magnetic iron oxide nanoparticles encapsulated in maleic acid-2-acrylamido-2-methyl-1-propanesulfonate based polymer. This composite nanoparticle is specified for the high-pressure/high-temperature (HPHT) oilfield scale inhibition application. The process includes a facile-ultrasound-supported addition reaction to obtain iron oxide nanoparticles with surface coated by oleic acid. Then via inverse microemulsion polymerization with selected monomers, the specifically designed copolymers have been formatted in nanoscale. The structure and morphology of obtained materials were characterized by transmission electron microscopy (TEM), x-ray powder diffraction (XRD), Fourier transform infrared spectroscopy (FTIR) and the thermal stability. The effectiveness of synthesized compounds as a carbonate scale inhibitor was investigated by testing method NACE standard TM 03-074-95 at aging temperature of 70, 90 and 120 °C. The magnetic nanocomposite particles can be easily collected and detected demonstrating their superior monitoring ability, which is absent in the case of conventional copolymer-based scale inhibitor.
Export citation and abstract BibTeX RIS

Content from this work may be used under the terms of the Creative Commons Attribution 3.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Secondary recovery is one of most important stages in crude oil production. Water, or brine in offshore mines, is most frequently used as a flooding agent due to its availability and low cost. However, the injection of water into the oil-bearing reservoir for maintaining the reservoir pressure, improving sweep efficiency and, consequently, increasing the recovery factor, is also the main cause of scale deposition. In flooded water, ions of sparingly soluble salts, notably CaCO3, CaSO4, Ca3(PO4)2, Mg(OH)2 and silica are contained. A potential for scale precipitation develops whenever process conditions lead to the creation of super-saturation with respect to one or more sparingly soluble salts [1,2]. The injected water will react with both the water already in the pore space of the rock (formation water), and with the minerals in the rock itself. This reaction will create scale formation. Scale deposition causes negative impacts on maintaining and exploiting gas and oil products as well as reducing the vital productivity of oilfields [1].
The growth of scales could be prevented or slowed down with the use of scale inhibitors. Polymer scale inhibitors are widely used to prevent scale formation in off-shore oil wells and in water treatment because of their environmental safety and high effectiveness [3]. In addition, the research and development of polymer scale inhibitors has been much promoted in recent years, mostly organic based carboxylic acids, sulfonic acids, phosphor-contained inhibitors and the so-called green inhibitors. These agents have important properties, such as the thermal stability, distinct solubility threshold effect, low dosage effect, the cooperative effect with other reagents and high- temperature endurance. Those polymers are water-soluble and widely used in the oilfield as scale inhibitors [4–6]. It is still challenging to monitor them from the water after being used in oilfields. Currently, the important requirement for oilfield scale inhibitors is to be sure when the next squeeze treatment has to be performed. To limit this drawback, it is common to add phosphonate based scale inhibitors into the polymers. However, today many countries have restricted or banned the use of the phosphonate inhibitors in offshore scale treatment [7].
Meanwhile, nano-sized magnetic polymer composites with core–shell structures provide many advantages in the field of basic research and industry because of the ability of magnetic nanoparticles to be controlled and the swelling capability of soluble polymer species [8,9]. To prepare the nanocomposite particles with core–shell structure, conventional and inverse mini-emulsion polymerization are mostly used with the high yield and conversion [10,11]. The mechanism in core–shell structure formation is polymer encapsulation happening on the surface of solid magnetic nanoparticles. Among various candidates, super magnetic Fe3O4 nanoparticles coated with polymers are most researched and applied due to their strong magnetic property and behavior, and also the relatively low toxicity to the human body and environment [6, 11–13].
In this paper we present the synthesis of magnetic nanocomposites based on the polymerization of maleic acid with 2-acrylamido-2-methylpropane sulfonic acid (AMPS) via inverse miniemulsion copolymerization in order to aim the combination of advantages of magnetic iron oxide nanoparticles and the scale control ability of sulfonate, carboxylate based copolymers. Sizes of the nanoparticles could be controllable under 100 nm. Finally, the scale control and detecting abilities were evaluated for the final nanocomposite product at high temperatures 70, 90 and 120 °C to see whether there appear outstanding advantages comparing with other conventional copolymer based scale inhibitors.
2. Experimental
2.1. Materials
Maleic anhydride, sodium 2-acrylamido-2-methyl-1-propanesulfonate (AMPS), ferrous chloride tetrahydrate (FeCl2·4H2O), ferrous chloride hexahydrate (FeCl3·6H2O), oleic acid (98%), ammonium hydroxide (30%), hydro chloride acid, sodium lauryl sulfate, cyclo hexane, sorbitan monooleate (Span-80) and disodium ethylenediaminetetraacetiate (EDTA) were purchased and used as-received from Merck Chemicals, azobisisobutyronitrile from Sigma Aldrich.
Carbonate and sulfate based seawater were prepared according to the NACE standard method TM 03-074-951 but modified to simulate the produced brine composition of the desired brines.
2.2. Procedure
2.2.1. Synthesis of oleic acid coated iron oxide nanoparticles (OMNPs).
Oleic coated iron oxide nanoparticles were synthesized by co-precipitation under ultrasound irradiation support. Briefly, FeCl2·4H2O (4.0 g, 0.02 mol), FeCl3·6H2O (12.96 g, 0.01 mol) were dissolved in distilled and deoxygenated water (200 ml), then ammonium hydroxide (120 ml) was quickly added to mixture under ultrasound irradiating at 20 kHz at room temperature. After 30 min, oleic acid (5 ml) was continuously added into the mixture of resulting black dispersion. The reaction temperature was raised to 91 °C in an hour. The modification was ended by adding 100 ml of HCl (0.01 M) in order to exclude base catalyst and unreacted oleic acid. Thereafter, the black dispersed particles were collected by a magnetic field and washed several times by distilled water till the pH of the washing water was around 7. Finally, the magnetic product was dried in an oven at 65 °C for 8 h. (figure 1).
Figure 1. The synthetic route of OMNPs.
Download figure:
Standard image High-resolution image2.2.2. Synthesis of OMNPs
encapsulated copolymer of maleic acid and sodium 2-acrylamido-2-methyl-1-propanesulfonate (MA-co-AMPS). MA-co-AMPS coated magnetic iron oxide nanoparticles were prepared via inverse mini-emulsion polymerization in a 250 ml three neck-jacketed reactor fitted with an ultrasound transmitter and mechanical stirrer under nitrogen atmosphere. Following a representative synthetic procedure for inverse mini-emulsion polymerization, 10.0 g of AMPS, 5.0 g of maleic anhydride were dissolved in 80 ml of distilled and deoxygenated water as a discontinuous phase. Then 5.0 g of magnetic iron oxide nanoparticles, 1.5 g of Span-80 were dissolved in 80 ml of cyclohexane. This aqueous solution was poured into the reactor. The mixture was ultrasound irradiated at 20 kHz and stirred at 400 rpm; the temperature was maintained at room temperature for 30 min. After that, the reaction temperature was raised to 70 °C; 150 mg of azobisisobutyronitrile in 2.0 ml of water was slowly injected to initiate polymerization. After 2 h of ultrasound irradiating and stirring, the composite product was taken out of the reactor. The resulting product was washed in an ethanol/water (9/1) solution and dried at 50 °C for 6 h.
We have implemented the similar synthetic route for MA-co-AMPS based copolymer nanoparticles without magnetic iron oxide inside in order to determine the scale control ability in preventing the precipitation of calcium carbonate and calcium sulfate from solution. (figure 2).
Figure 2. Preparation of OMNPs encapsulated copolymer of maleic acid and sodium 2-acrylamido-2-methyl-1-propanesulfonate (MA-co-AMPS).
Download figure:
Standard image High-resolution image2.2.3. Scale inhibition test.
The experiments were performed with various used doses of scale inhibitors; active concentrations of 5, 10, 20 ppm, and mixed with feed solution at 70, 90 and 120 °C for 24 ageing hours. After that, calcium carbonate precipitates were collected and identified in the crystal morphology by TEM and XRD. Free calcium ion from the scale tests was titrated with ETDA and evaluated on the calcium inhibition efficiency (ICa).
Feed solution (carbonate based feed solution) was prepared by mixing solution A containing the cationic components and the anionic components of the solution B as described in table 1. The active ionic concentration in each solution was determined by an ionic titration.
Table 1. Components of feed solution.
| Components of the solution A for 500 ml | Net weight (g) | Active cationic concentration (mol l−1) | Active anionic concentration (mol l−1) |
|---|---|---|---|
| CaCl2·2H2O | 6.075 | [Ca2+]=0.0826 | |
| MgCl2·6H2O | 1.840 | [Mg2+]=0.0180 | |
| NaCl | 16.505 | ||
| NaHCO3 | 3.682 | [HCO3−]=0.0876 | |
| Na2SO4 | 0.0147 | ||
| NaCl | 16.524 |
3. Results and discussion
3.1. Synthesis of oleic acid coated iron oxide nanoparticles (OMNPs)
The magnetic iron oxide nanoparticles were synthesized by co-precipitation of Fe2+ and Fe3+ ions in an aqueous solution upon addition of ammonium hydroxide and ultrasound irradiation assisted. Due to strong magnetic interactions, the MNPs tend to aggregate in order to reduce their surface energy. In this work, oleic acid was coated on the MNP surface to stabilize and enhance the activeness of obtained MNPs for a further reaction of polymerization. It is well known that oleic acid provides a high affinity with iron oxide through the chemical interaction between their –COO–groups and Fe atoms. As a result, the hydrophobic tails of oleic acid molecule face outwards and generate a non-polar shell.
The presence of oleic acid on the surface of MNPs was confirmed by FTIR (figure 3). The bands at 2862 and 2923 cm−1 were observed according to the stretch modes of –CH2– and –CH3 of oleic acid. The stretching vibration of  at 1710 cm−1 was clearly detected, and the band at 1438 cm−1, 1518 cm−1 were clearly recognized and attributed to the asymmetric and symmetric stretching vibrations of –COO–functional group, indicating that the layer of oleic acid was successfully coated on the surface of MNPs. Furthermore, the band at 587 cm−1, corresponding to the vibration of the Fe–O bonds in the structure of Fe3O4 was observed. The results corresponded well with other previous data.
at 1710 cm−1 was clearly detected, and the band at 1438 cm−1, 1518 cm−1 were clearly recognized and attributed to the asymmetric and symmetric stretching vibrations of –COO–functional group, indicating that the layer of oleic acid was successfully coated on the surface of MNPs. Furthermore, the band at 587 cm−1, corresponding to the vibration of the Fe–O bonds in the structure of Fe3O4 was observed. The results corresponded well with other previous data.
Figure 3. FTIR spectrum of oleic acid coated magnetic iron oxide nanoparticles.
Download figure:
Standard image High-resolution image3.2. Synthesis of OMNPs encapsulated maleic acid (MA) co sodium 2-acrylamido-2-methyl-1-propanesulfonate (AMPS) copolymers (MA-co-AMPS-OMNPs)
The MA-co-AMPS-OMNPs composite was prepared by inverse mini-emulsion polymerization of OMNPs, MA and AMPS. Using this procedure, the formation of core–shell structure can be made through three possible principles: (i) well dispersion by ultrasound irradiation assistance; (ii) electrostatic interactions between OMNPs with NH-, SO3−,–COO− of maleic acid and AMPS and (iii) the functional group of oleic acid on the surface of OMNPs not only makes the OMNPs more stable but it can also be a temporary monomer in the polymerization reaction.
The final product was characterized by FTIR, TEM and XRD.
The FTIR spectroscopy from figure 4 was employed to characterize the chemical functional groups present in the MA-co-AMPS-OMNPs composite comparing to those of the original MNPs, OMNPs, MA-co-AMPS copolymer. The FTIR spectrum of the original MA-co-AMPS copolymers showed the characteristic signals at 3200–3500 cm−1 (–NH–stretching), 2928 cm−1 (alkyl- stretching), 629 cm−1 (S–O stretching) 1116 cm−1 ( stretching) and 1033 cm−1 (C–S stretching) present in the chemical structure of AMPS. In addition, the signal at 1665 cm−1 and 1189 cm−1 belong to
stretching) and 1033 cm−1 (C–S stretching) present in the chemical structure of AMPS. In addition, the signal at 1665 cm−1 and 1189 cm−1 belong to  and C–O stretching vibrations of maleic acid in copolymer structure. As we can see, the FTIR spectrum of the composites seemly presents all the signals of the original components, including those at 3344 cm−1 (NH–stretching vibration), 2923 cm−1 and 2862 cm−1 (CH– and CH3–stretching vibration), 1711 cm−1 and 1537 cm−1 (
and C–O stretching vibrations of maleic acid in copolymer structure. As we can see, the FTIR spectrum of the composites seemly presents all the signals of the original components, including those at 3344 cm−1 (NH–stretching vibration), 2923 cm−1 and 2862 cm−1 (CH– and CH3–stretching vibration), 1711 cm−1 and 1537 cm−1 ( stretching vibration), 1039 cm−1 (
stretching vibration), 1039 cm−1 ( signal), 632 cm−1 (C–S signal), 581 cm−1 (Fe–O signal).
signal), 632 cm−1 (C–S signal), 581 cm−1 (Fe–O signal).
Figure 4. FTIR spectrum comparison of (a) magnetic iron oxide nanoparticles; (b) oleic acid coated magnetic iron oxide nanoparticles; (c) maleic-co- acrylamido-2-methyl-1-propanesulfonate copolymers; (d) magnetic iron oxide nanoparticles encapsulated maleic-co- acrylamido-2-methyl-1-propanesulfonate copolymers.
Download figure:
Standard image High-resolution imageFigures 5 and 6 demonstrate the TEM micrographs of OMNPs and MA-co-AMPS- OMNPs, respectively. In both micrographs, MNPs and MA-co-AMPS- OMNPs were spherical in shape with the average size of 20–30 nm. The MNPs monodispersed particles were better obtained and observed than MA-co-AMPS-OMNPs particles due to the amorphous structure of the copolymer on the surface of composites and the adhesive ability of this composite is essentially higher than those of MNPs and OMNPs.
Figure 5. TEM of OMNPs.
Download figure:
Standard image High-resolution imageFigure 6. TEM of MA-co-AMPS-OMNPs.
Download figure:
Standard image High-resolution imageIn order to identify and interpret the morphology of the composite product, XRD spectra of the samples were taken and the results were shown in figure 7. In figure 7, XRD spectrum related to the composite which revealed that the crystallographic structure of MNPs present in MA-co-AMPS copolymer matrix after polymerization did not change compared to that in OMNPs. In addition, XRD spectrum of composite shows the low intensity peak at 2θ less than 25° which is related to the presence of the polymer in the nanocomposites. The presence of the peak at 30°, 36° and around 40° can exclude the existence of magnetic iron oxide.
Figure 7. XRD patterns of (a) magnetic iron oxide nanoparticles encapsulated maleic-co- acrylamido-2-methyl-1-propanesulfonate copolymers (composite) and (b) magnetic iron oxide nanoparticles (Fe3O4-OLD).
Download figure:
Standard image High-resolution imageBasing on XRD data we can calculate the average crystallite size of obtained particles from the Debye–Scherres equation existence

where D is average crystallite size (Å), λ is x-ray wavelength (Cu-Kα, λ = 1.5418 Å), β is full-width at half-maximum and θ is the Bragg diffraction angle. As calculated, the average size of obtained OMNPs and MA-co-AMPS-OMNPs particles were at the range of 20–30 nm. These results were similar to those in TEM micrographs.
Thermal characteristics of coated iron oxide nanoparticles was investigated by thermogravimetric analyzer (TGA) and the resultant thermograms were presented in figure 8. The composite product showed a considerable thermal stability under inert conditions. At above 250 °C, a remarkable weight loss derives from the thermal decomposition of the side groups and polymer backbone and it continues up to 900 °C. This thermogravimetry (TG) analysis showed that weight percentages of inorganic Fe3O4 and polymeric constituents are 68 and 32%, respectively.
Figure 8. TGA patterns of composite.
Download figure:
Standard image High-resolution image3.3. Scale inhibition test
According to previous studies [14 ,15], it is strongly believed that the inhibitor molecules must be adsorbed at the active growth sites on the surface, which may be crystal defects, thus preventing further crystal growth by interfering with the growth process. The inhibition of scale formation is affected by both the location of the adsorbed inhibitor at the crystal surface, and the extent of chemical bonding with the surface. Therefore, the scale efficiency is determined by the ability of complex creation between the scale inhibitors and the multivalence cations of the seed solution.
In terms of morphology, with the absence of an inhibitor, obtained calcium carbonate precipitates were in calcite crystal form with more angular shapes, very high adhesion surface and were difficult to wash out from the deposited surface. In contrast, in the presence of inhibitors, calcium carbonate crystals were obtained in the form of agaronite with smooth surfaces and easily washed away with hydraulic lines or other washing methods.
In our study, calcium carbonate crystals obtained from testing samples got similar results, with the presence of inhibitors based on copolymers or composites; obtained calcium carbonate precipitates were also in agaronite form, but when inhibitors were absent, in calcite forms. Both pure polymers and composites had the same impact on the morphology of the obtained calcium carbonate crystals through similar mechanisms which were previously mentioned (figures 9 and 10).
Figure 9 TEM image of agaronite crystals.
Download figure:
Standard image High-resolution imageFigure 10 TEM image of calcite crystals.
Download figure:
Standard image High-resolution imageCalcium inhibition efficiency was determined according to the NACE standard TM 03-074-95 testing method. (See footnote 1). Inhibition efficiency is calculated according to the following equation:
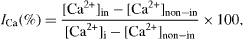
where ICa(%) is calcium inhibition efficiency, [Ca2+]in is soluble calcium concentration of inhibited sample, [Ca2+]non−in is soluble calcium concentration of the uninhibited sample and [Ca2+]i is initial soluble calcium concentration.
Soluble calcium concentrations are obtained by sample titration with standard EDTA solution and ammonium purpurate indicator. The results are shown in the charts below.
As shown in figures 11 and 12, at 90 °C and 5 ppm of used dose, ICa of inhibitor based on composite and polymer products reached highest values. Namely, the best ICa value was 63.64% accounting for composites and 65.00% for pure copolymers. At 120 °C, ICa of copolymers was likely higher than those of composites but not much: 22.22 and 25.03% attributing to composite and copolymers, respectively. This result could be related to the unusual high molecular weight of composite, due to heavy Fe3O4 core, compared to pure polymers. So that the same concentrations, the active copolymer component contained in composite samples was obviously less than those of copolymers.
Figure 11. Graphics of ICa of composites.
Download figure:
Standard image High-resolution imageFigure 12. Graphics of ICa of copolymers.
Download figure:
Standard image High-resolution imageBesides the inhibition activity of magnetic composites as high as that of the parent copolymer, the most promising advanced characteristic of this new inhibitor is that it can be easily detected in produced water by magnetic monitoring devices after being used in oilfields. This is a very important requirement for oilfield scale inhibitors, and so far do not have any commercial polymer based scale inhibitors that meet the requirement. For this reason, we should try to further synthesize and develop effective magnetic inhibitors.
4. Conclusion
Oleic coated magnetic iron oxide nanoparticles were synthesized with the average size distribution of 20–30 nm and well identified structure and morphology. Via inverse mini-emulsion, polymerization of the magnetic iron oxide nanoparticles encapsulated maleic co acrylamido-2-methyl-1-propanesulfonate copolymers (magnetic composites) with well identified structure and characteristics have been synthesized.
At 90 °C, with concentration of 5 ppm, magnetic composites could inhibit carbonate scale deposition up to 63.64%. The specific advantage of this composite scale inhibitor is the possibility of being easily monitored from produced water by a magnetic device.
Footnotes
- 1
NACE standard TM 03-04 2005 Laboratory test to determine the ability of scale inhibitors to prevent the precipitation of calcium sulfate and calcium carbonte from solution Natl. Assoc. of Corros. Eng. Houston, Texas.












