Abstract
Gold nanoparticles (AuNPs) were synthesized using two different mono-deoxynucleosides, namely, deoxycytidine (dC) and deoxyadenosine (dA) and the size of the nanoparticles in aqueous dispersions was measured to be approximately 10 and 23 nm, respectively. It was also observed that the AuNPs, synthesized using deoxycytidine (dC), self-assembled to a stable cauliflower-type structure of size approximately 230 nm over a long period of ageing, during which the solution colour was seen continuously changing from pale yellow to deep green. The self-assembly of dC–Au nanoparticles (dC–AuNPs) with time was investigated using UV–visible spectroscopy and dynamic light scattering (DLS) techniques. We have also observed that the self-assembly of dC–AuNPs was dependent on the solution pH; i.e. the aggregates could be dissociated and re-associated upon varying the solution pH which we assumed to be due to breaking and forming of hydrogen bonds between  and
and  groups of dC among the neighbouring dC–AuNPs. In contrast, AuNPs synthesized using deoxyadenosine (dA–AuNPs) were quite stable in aqueous medium.
groups of dC among the neighbouring dC–AuNPs. In contrast, AuNPs synthesized using deoxyadenosine (dA–AuNPs) were quite stable in aqueous medium.
Export citation and abstract BibTeX RIS

Content from this work may be used under the terms of the Creative Commons Attribution 3.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Reversible aggregation or self-assembly of metal colloids tuned by the bio-molecular/macromolecular interactions [1–5] tethered on the surface of the nanoparticles is of central interest in the research community, due to its potential practical applications such as sensing [6, 7], DNA hybridization [8–11] and cellular uptake [12, 13]. In particular, oligonucleotides functionalized gold nanoparticles (AuNPs) [14–19] show promising results in tuning and controlling the patterns of assemblies [17] and crystallization [20–22] by effectively engineering the sequential information, DNA linker's length [22], etc. The concept of programmable colloidal crystallization was first originated by Mirkin and co-worker [24], its credit lies behind the exploitation of non-covalent interactions, which gives an immense control in tuning the self-assembly. Until then these powerful and challenging ideas had been extensively probed due to the fact that the DNA–gold interaction plays a potential role in widespread applications. These sequence-specific assemblies of AuNPs act as powerful diagnostic devices [23–26]. Biancaniello et al [27] reported the direct measurement of the DNA induced interactions between colloidal microspheres leading to assembled colloidal crystals using optical tweezers and an exact theoretical framework for their experiments addressing the assemblies mediated by weak adhesions. Leunissen et al [28] demonstrated the possibility of finely tuning the DNA-functionalized colloids thereby gaining control over their self-assembly. It is now also possible to program the crystallization of the dispersed colloidal assemblies by carefully engineering the sequences of the linker DNAs thereby controlling their interactions [21, 22]. As for the growing interest in the DNA–nanoparticle interactions, it also becomes important to understand the effect of nucleobases on inducing the self-assembly of AuNPs. Recently, Storhoff et al [29] investigated the binding affinity of (5–20 base) deoxynucleoside on citrate stabilized AuNPs, pertaining to the fact of ascertaining the effects of nucleosides on the self-assembly of AuNPs. They also investigated the stability of the poly-deoxynucleoside immobilized AuNPs upon salt addition. So far, there is no report in understanding the effects of mono-deoxynucleoside on the synthesis and self-assembly of AuNPs.
We report here one-pot synthesis of water dispersible AuNPs using two deoxynucleosides, namely, deoxycytidine (dC) and deoxyadenosine (dA) and also the self-assembly of as-synthesized AuNPs with time. This bypasses the need for a separate functionalization process [30], since the immobilization of molecules (dC and dA) on the surfaces of AuNPs took place during the synthesis process and provided the Stern layer to the surfaces of nanoparticles. We have also investigated the self-assembly of AuNPs in the aqueous medium at different solution pH.
2. Materials and methods
2.1. Materials and synthesis of AuNPs
Deoxycytidine (C9H13N3O4 ⩾ 90%) and deoxyadenosine (C10H13N5O3 ⩾ 99%) were purchased from Sigma. Choloroauric acid (HAuCl4 ⩾ 97%) was purchased from Loba Chemie. Sodium hydroxide pellets (NaOH ⩾ 98%), hydrochloric acid (HCl) and nitric acid (HNO3) were purchased from Merck. All the glass wares utilized in the experiments were extensively washed with aquaregia in order to remove any pervious adsorbed metal particles and with double distilled water several times, and finally rinsed with milli-Q water (18.2 mΩ). Milli-Q water was used throughout the experiments. All the chemicals were used without any further purification.
A novel and facile one-pot route for synthesis of water-soluble AuNPs using single deoxynucleoside has been reported. Briefly, HAuCl4 a stock solution of specific concentration was prepared and was isolated from light, in order to prevent any photo-induced reduction. The preserved metal salt solution was put on a temperature-controlled heater and was kept undisturbed at 150 °C. Within seconds, refrigerated dC solution (1 mM) was gently added, so that the concentration of HAuCl4 in aqueous solution became 0.12 mM, and homogenized by gentle shaking. This mixture was left for heating, say about 15 min at 150 °C. During the reaction process the solution colour was transformed from very light yellow to deeper yellow in the case of reaction with dC; on the other hand, the solution colour changed from light yellow to orange while dA was used as the reaction agent. The as-synthesized AuNPs, obtained using dC and dA, will be referred as dC–Au and dA–Au throughout the report.
The as-synthesized AuNPs aqueous dispersions were kept in a dark environment. Interestingly, the AuNPs synthesized using dC (dC–Au nanoparticles) showed a spontaneous change in the solution colour as yellow → greenish yellow → green without any precipitation, indicating the self-assembly of AuNPs to bigger clusters. The self-assembled clusters of dC–AuNPs will be referred as dC-AuC. On the other hand, the AuNPs synthesized using dA (dA–AuNPs) were found to be stable for months without any change in the solution colour.
2.2. Methods
The surface characterization of AuNPs was carried out using Fourier transform infrared (FTIR) spectrophotometer, Perkin Elmer (USA) with resolution ±1.0 cm−1. Dried powder of AuNPs and KBr were mixed and pelletized for FTIR measurements. The zeta potential measurements for AuNPs dispersions were carried out using Zetasizer Nano ZSP, Malvern (UK). The measurements were in regular time intervals to monitor the change in zeta potential of AuNPs dispersions. The UV–visible spectrophotometer, Shimadazu UV 2470, with a quartz cuvette of 1 cm path length was employed for optical absorbance measurements. The dynamic light scattering (DLS) measurements were performed using a home-made setup [31, 32] at 90° scattering angle and data were analysed using the CONTIN algorithm [32] to determine the hydrodynamic diameter (DH) of AuNPs. Direct images at different stages of self-assembling of dC–AuNPs were obtained using a high-resolution transmission electron microscope (HRTEM, FEI Technai G230). X-ray diffraction (XRD) patterns of dried powders of dC–AuNPs, dA–AuNPs and dC–AuC were obtained using the Bruker D8 Advance.
3. Results and discussion
We have synthesized AuNPs using two different mono-deoxynucleosides, namely, deoxyadenosine, i.e. C10H13N5O3 (dA) and deoxycytidine, i.e. C9H13N3O4 (dC). In scheme
Scheme 1. Molecular structure of (a) deoxyadenosine and (b) deoxycytidine.
Download figure:
Standard image High-resolution image3.1. FTIR study
Figure 1(a) shows the FTIR spectra of dA (filled squares) and dA–AuNPs (open squares) and figure 1(b) shows those of dC (filled circles) and dC–AuC (open circles). Figure 1(a) shows prominent peaks within 1500–1800 cm−1 representing the exocyclic amino region of dA (see scheme 1(a)). In the case of dA–AuNPs, reduction of the intensity of the peak at 1654 cm−1, which is assigned to NH2 scissoring, might indicate that the respective functional group is responsible for the reduction of the metal salt. The absence of 1604 cm−1 peak, which is the combination of NH2 and  scissoring and stretching vibrations [33], in dA–AuNPs spectrum indicates that dA might adsorb onto the AuNP surface through
scissoring and stretching vibrations [33], in dA–AuNPs spectrum indicates that dA might adsorb onto the AuNP surface through  as reported [34]. Similarly, dC–AuNPs were surface functionalized with dC (figure 1(b)), which has further instigated the self-assembling of dC–AuNPs to bigger clusters, i.e. dC–AuC, in aqueous medium at pH ∼ 6.5. The absence (or a very weak presence) of the absorption peak at 1616 cm−1, which corresponds to the bending vibrations of
as reported [34]. Similarly, dC–AuNPs were surface functionalized with dC (figure 1(b)), which has further instigated the self-assembling of dC–AuNPs to bigger clusters, i.e. dC–AuC, in aqueous medium at pH ∼ 6.5. The absence (or a very weak presence) of the absorption peak at 1616 cm−1, which corresponds to the bending vibrations of  , indicates that NH2 played an important role in the synthesis of dC–AuNPs. A shift of the peak at 1292 cm−1, which corresponds to the pyrimidine ring of dC (see scheme 1(b)), towards longer wavenumber region indicates that it might adsorbed on to the AuNP surface, as reported [35].
, indicates that NH2 played an important role in the synthesis of dC–AuNPs. A shift of the peak at 1292 cm−1, which corresponds to the pyrimidine ring of dC (see scheme 1(b)), towards longer wavenumber region indicates that it might adsorbed on to the AuNP surface, as reported [35].
Figure 1. FTIR spectra of (a) dA (▪) and A–AuNPs (□), (b) dC ( ) and dC–AuNPs (
) and dC–AuNPs ( ).
).
Download figure:
Standard image High-resolution image3.2. UV–visible study
Figure 2 shows the surface plasmon resonance (SPR) absorption peaks of as-synthesized dC–AuNPs and dA–AuNPs dispersions around 534 and 538 nm, respectively. Insets of figure 2 show the photos of as-synthesized dispersions.
Figure 2. SPR of as-synthesized (- -) dC–Au and (-▪-) dA-Au. Insets show digital photos of dC–AuNPs dispersion (left) and dA–AuNPs dispersion (right).
-) dC–Au and (-▪-) dA-Au. Insets show digital photos of dC–AuNPs dispersion (left) and dA–AuNPs dispersion (right).
Download figure:
Standard image High-resolution imageThe SPR peak position λSPR of dC–AuNPs was found to shift with time towards longer wavelength regime up to around 628 nm (figures 3(a)–(c)), indicating the self-assembling process and the formation of stable aggregates of cauliflower type structures in the dispersion. As a consequence, the colour of dC–AuNPs dispersion was seen to vary spontaneously from pale yellow to deep green (insets of figure 3(c)). The final dispersion was stable for a long period (see figure S1 of supplementary information, available from stacks.iop.org/ANSN/4/045014/mmedia). On the other hand, dA capped AuNPs (dA-AuNPs) dispersion did not show any change in the SPR absorption peak position over a period of one month or more, indicating no aggregation (figure S2 of supplementary information) took place in the as-synthesized dispersion. It has also been observed that dA–AuNPs were spherical in morphology of size around 23 nm and were sterically capped by dA, which has acted as both a reducing and a stabilizing agent (see figure S4(a) of supplementary information).
Figure 3. (a), (b) SPR peaks of dC–AuNPs dispersion observed at different time intervals; (c) plot (○) shows the variation of λSPR as a function of time; (d) cartoons show the possible mode of self-assembly of dC–AuNPs, by forming hydrogen bonds (dashed lines) between  and
and  of neighbouring dC–AuNPs, to cauliflower type clusters with time.
of neighbouring dC–AuNPs, to cauliflower type clusters with time.
Download figure:
Standard image High-resolution imageAs discussed earlier, each dC has one oxygen (O) atom in the benzene ring (scheme 1(b)) which is capable of developing a hydrogen bond with the  group of the neighbouring dC–AuNPs, and hence, forming a cascade structure (dC–AuC) as shown by a cartoon in figure 3(d). Hydrogen bonds are weak, and therefore, the self-assembled cascade structure can be easily broken.
group of the neighbouring dC–AuNPs, and hence, forming a cascade structure (dC–AuC) as shown by a cartoon in figure 3(d). Hydrogen bonds are weak, and therefore, the self-assembled cascade structure can be easily broken.
3.3. DLS and TEM studies
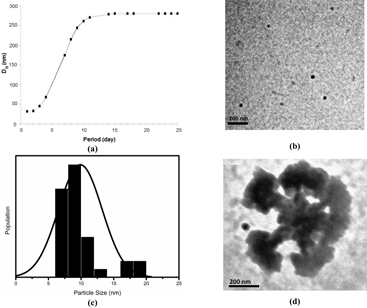
Using DLS we have investigated change in the particle size (DH) with time, as a consequence of aggregation, in both dC–AuNPs and dA–AuNPs dispersions. Figure 4(a) shows the increase of DH of dC–AuNPs with time from 31 to around 260 nm, indicating the self-assembly of as-synthesized nanoparticles with time to a stable structure. The kinetics of self-assembly was sluggish. The DH of dA–AuNPs was also measured using DLS and found almost unchanged with ageing time (figure S2(b) of supplementary information, available from stacks.iop.org/ANSN/4/045014/mmedia).
Figure 4. (a) Variation of DH of dC–AuNPs as function of time indicates the self-assembly of AuNPs; (b) and (c) TEM micrograph of as-synthesized dC–AuNPs and the corresponding histogram showing the mean size ∼10 nm (σ = 3.3); (d) TEM micrograph of the stable cauliflower type gold clusters (dC–AuC).
Download figure:
Standard image High-resolution imageUsing TEM we have investigated the size of dC–AuNPs and dC–AuC. Figure 4(b) shows the image of as-synthesized dC–AuNPs and figure 4(c) shows the histogram obtained from TEM image of as-synthesized dC–AuNPs. The average size was found to be around 10 nm which further self-assembled to the stable cauliflower type structure (dC–AuC) of size ∼235 nm in around 25 days (figure 4(c)). This is in consistency with the DLS result. The intermediate stages of assemblies were also monitored using TEM and was found systematic (see figure S3 of supplementary information). XRD patterns for dried dC–AuNPs and dC–AuC were also taken (see figure S5 of supplementary information) which showed the presence of Au (111) peak.
3.4. Zeta potential measurement
We have measured the zeta potential of each dispersion to investigate the stability of the dispersion and given the result in table 1. The zeta potential of the dC–AuNPs dispersion was very low (∼ 0.75 mV), indicating that the as-synthesized dC–AuNPs were unstable and formed self-assembled bigger clusters (dC–AuC) of size ∼235 nm. The zeta potential of the aggregates was high (∼ 32 mV) indicating the stabilization of the dispersion. On the other hand, the zeta potential of the as-synthesized dA–AuNPs dispersion was high (∼ 29 mV) revealing that the dispersion was stable, as we have discussed in earlier sections.
Table 1. Zeta potential values at different conditions of the sample.
| Conditions of the samples | ζ-potential (mV) in dC–Au dispersion | ζ-potential (mV) in dA–Au dispersion |
|---|---|---|
| As-prepared (pH ∼7) | 0.747 | 29.0 |
| 3 days aged | 2.90 | 29.0 |
| 12 days aged | 7.24 | 29.0 |
| 21 days aged | 32.2 | 29.0 |
| at pH ∼9.8 | −34.3 | Not measured as |
| at pH ∼10.9 | −11.7 | there was no visual change |
| By reverting back to the original pH (i.e. ∼7) | −11.7 | in the dispersion colour. |
3.5. Effect of solution pH and temperature on dC–AuC
It was observed that optical property of the dC–AuC dispersion could be varied by changing the pH. Change of the self-assembled structure by changing the dispersion pH was studied by observing the shift in SPR peak position of dC–AuNPs dispersion, as well as by measuring the change of DH in the corresponding DLS measurements. Interestingly, the clustering was found to be reversible on both sides of the neutral pH (∼ 6.5) of dC–AuC dispersion.
The SPR position shifts towards lower wavelength side, around 530 nm at both acidic and basic pH and again returns back to 628 nm when the pH of the solution was brought back to neutral. This indicates that a slight variation in the solution pH (figures 5(a) and (b)) could break the hydrogen bonds between  and
and  of neighbouring dC–AuNPs (as discussed in earlier section). This kind of pH dependent self-assembling of nanoparticles can be utilized for advanced delivery application in cancer therapy [12].
of neighbouring dC–AuNPs (as discussed in earlier section). This kind of pH dependent self-assembling of nanoparticles can be utilized for advanced delivery application in cancer therapy [12].
Figure 5. (a), (b) Absorbance spectra showing peak shifts indicative of dissociation and re-association of dC–AuC at different pH; (c): change of DH of dC–AuC with pH.
Download figure:
Standard image High-resolution imageIt is to be noted that there are very feeble signatures of other plasmon modes at different solution pH corresponding to few intermediate structures of unknown size of dissociating (or re-associating) clusters. The decrease of cluster size of dC–AuC with the solution pH was also investigated using DLS. Figure 5(c) shows the variation of DH of the cluster with variation of pH from 6 to 12.
We have also observed that upon heating above 35 °C, the green dC–AuC dispersion changed to the yellow dispersion which remained stable for a long period of time, indicating that clusters were permanently dissociated, and no further clustering was taking place.
We have generated nanoparticle–nanoparticle interaction plots for dC–AuNPs dispersion at different ageing times using the following Derjaguin, Landau, Verwey and Overbeek (DLVO) expression [36]

here x is the distance between the NPs. Considering the case of κa > 1, where κ is the inverse of the Debye length, and a is the particle radius, the electrostatic repulsion can be expressed as

where ε is the permittivity of the solvent and ϕo is the surface potential of the particles and the Debye parameter  , where c is the concentration of the z valent ionic species and e is the charge of proton or electron. The simplified van der Waals attractive potential can be written as
, where c is the concentration of the z valent ionic species and e is the charge of proton or electron. The simplified van der Waals attractive potential can be written as

In the calculation, z for dC–AuNPs has been taken as 1; ϕo has been taken as the experimental values of ζ-potential for as-synthesized and 12 days aged dC–Au dispersions. The Hamakar constant A has been taken as kBT (in joules) [36]. The interaction energy versus particle–particle distance for dC–AuNPs before and after ageing for 12 days (obtained using equation (1)) is plotted in figure 6. It is clear that the interaction potential was attractive (or weakly repulsive) in as-synthesized dC–AuNPs dispersion and caused self-assembly, as mentioned in previous sections. The self-assembly was automatically controlled by the interaction sites of dC molecules on nanoparticles surfaces. The interaction potential subsequently became more repulsive as ageing time for dC–Au dispersion increased, and appeared constant after forming stable cauliflower-type clusters.
Figure 6. DLVO potential for as-synthesized (solid line, bottom curve) and for 12 days aged (filled circles, top curve) dC–Au NPs dispersions.
Download figure:
Standard image High-resolution image4. Conclusion
We have reported a novel and facile method of synthesis of AuNPs using mono-deoxynucleosides, namely, deoxycytidine (dC) and deoxyadenosine (dA). The AuNPs dispersions were characterized using the UV–V is spectroscopy by examining the formation of SPR band. The as-synthesized nanoparticles were spherical of size approximately 10 and 23 nm in dC–Au and dA–Au dispersions, respectively. The self-assembly of AuNPs induced by the dC was studied using absorbance spectrum and DLS, and was found to be sensitive to the solution pH. The as-synthesized AuNPs (dC–AuNPs and dA–AuNPs) as well as self-assembled structures (dC–AuC) were studied using HRTEM. The self-assembled structure of dC–AuNPs (i.e. dC–AuC) was found to be cauliflower-type which was derived by the hydrogen bonding between  and
and  groups of dC molecules of the nearest neighbour dC–AuNPs. Using DLVO calculation we have shown that the particle–particle interaction in dC–AuNPs dispersion was attractive (unstable dispersion) at as-synthesized stage and became repulsive (stable dispersion) after ageing. In contrast, dA–AuNPs dispersion was stable and there was no change in ζ-potential, SPR peak position and particle size as measured at different times.
groups of dC molecules of the nearest neighbour dC–AuNPs. Using DLVO calculation we have shown that the particle–particle interaction in dC–AuNPs dispersion was attractive (unstable dispersion) at as-synthesized stage and became repulsive (stable dispersion) after ageing. In contrast, dA–AuNPs dispersion was stable and there was no change in ζ-potential, SPR peak position and particle size as measured at different times.
Acknowledgments
One of the author ANA thanks UGC, Meritorious Fellowship Scheme for the fellowship. Authors thank Mr Nitthin for his help in taking IR measurements. Authors also thank UGC CSR Collaborative Research Scheme for the Project CRS-M-165, in which this work was performed.