Abstract
Rutile TiO2 was synthesized by sol–gel method. Vanadium-doped rutile TiO2 nanoparticle was obtained by reactive grinding method. The photocatalytic activity was evaluated by the degradation of methylene blue (MB) under solar light at room temperature. The results show that after 4 h of milling the particle size of rutile decreased from 130 to 14 nm and the Brunauer–Emmet–Teller (BET) specific surface area increased from 7.18 to 15.12 m2 g−1. The vanadium doping promoted the particle growth and the particle size of vanadium-modified rutile TiO2 obtained by 4 h of milling is about 22 nm, but the BET specific surface area increased from 15.12 m2 g−1 for TiO2 to 20.8 m2 g−1 for vanadium-doped TiO2 under the same conditions. The 5% vanadium-doped rutile possessed better absorption ability of solar light; the calculated band gap energy value is 2.7 eV. The degradation rate of MB on vanadium-doped rutile TiO2 was higher than that of pure rutile obtained after the same time of milling.
Export citation and abstract BibTeX RIS

Content from this work may be used under the terms of the Creative Commons Attribution 3.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Among various semiconductors, TiO2 is the most promising photocatalyst because it is biologically and chemically inert; its band gap and position of valence band (VB) and conduction band (CB) are appropriate for the photocatalytic oxidation and reduction of a wide range of environmental pollution as well as for water splitting to generate hydrogen and oxygen [1–3]. However, because the band gap of anatase is 3.2 eV, TiO2 anatase only shows photocatalytic activities under the UV light of wavelength λ < 387 nm; the band gap of rutile is 3.0 eV and TiO2 rutile exhibits activity in a very small visible range near to the UV one with wavelength λ ⩽ 413 nm. So TiO2 can utilize only a small UV fraction of the solar light, about 2–5%. Many publications have focused on doping TiO2 by metals or non-metals, so that its photocatalytic activities exhibit in the visible range of light [4–9]. The authors show that activities of materials depend on their surface properties such as particle size, specific surface area, surface structure and active reaction sites. Those properties are dependent on the methods of preparation. The authors [4, 8, 10] indicated that Cr-doped TiO2 prepared by an impregnation method exhibits photocatalytic activities less than those of the Cr ions implanted TiO2. A majority of publications concentrates on modifying of TiO2 anatase. Since they demonstrated that the density of TiO2 octahedral in anatase is smaller than that in rutile (creating more hollow spaces inside the anatase for the process of transportation and diffusion of photogenerated holes and photogenerated electrons) and the anatase can be obtained at low temperature (<500 °C) with small particle size, the TiO2 production for application purposes requires controlling over reaction conditions to obtain anatase. When the preparation temperature is increased, not only is there a rise in the particle size but the anatase also converts into rutile. This means we always obtain TiO2 rutile with large size under normal calcination at high temperature and hence the photocatalytic activities of rutile are less than those of anatase.
In fact, the most common and stable form of TiO2 is rutile. If the rutile form of TiO2 can be utilized as a photocatalyst, the cost can be reduced, and also too much concern about controlling the conditions during production is not necessary.
For these above reasons, by the special method, we prepared TiO2 rutile with sufficiently small size and doping TiO2 with metal or metalloid ions can be expected to result in the total effects that decrease the band gap of material. According to the published works [7, 11], doping V2O5 into TiO2 anatase can reduce the band gap, shifts the absorption wavelength of the material to the visible range, thus, their photocatalytic activities significantly increase. Some authors have made an effort to obtain rutile and modified rutile by in situ hydrothermal method and they show effective catalytic properties [12].
By the top-down preparation route, Kaliagine et al [13] received many complex nano-oxides as catalysts using reactive grinding method. In the grinding process, solid reactions occurred by high energy and nanometer particles were created. So, in this work, we used this grinding reactive method for vanadium-doped rutile TiO2 and prepared a product that has high photocatalytic activities.
2. Experimental
According to [7, 9], the increase of vanadium doping promoted the particle growth, and enhanced 'red-shift' in the UV–Vis absorption spectra. It was found that the samples containing 2.5–5% of V2O5 have the highest degradation of MB. Accordingly, in the present work we prepared 5% V2O5/TiO2 rutile and studied its characterizations and photocatalytic activity.
2.1. Preparation of TiO2 rutile
Titanium butoxide and butanol were mixed together, then this mixture was dropped slowly into 3 M HCl solution. The obtained sol was stirred continuously for 4 hours and gel was created. The gel was dried at 120 °C for 48 h and calcinated at 750 °C for 4 h. TiO2 rutile powder was obtained.
2.2. Preparation V2O5/TiO2 rutile
The 5% V2O5/TiO2 was prepared by reactive grinding as follows: the milling process uses mixed powders of 5% of vanadium oxide V2O5 (Aldrich) and 95% of TiO2 rutile obtained above as follows. The powder of V2O5 and TiO2 was dried at 90 °C for 2 h to eliminate the water before weighing. TiO2 and the oxide mixture of (5% V2O5 + 95% TiO2) were mixed and introduced in two vials with the volume of 65 ml and two types of balls of 1/2 and 1/4 inch. The vials and the balls are made with tempered steel material. The milling process was performed in a SPEX 8000D shaker mill for milling time tmil of 2, 4 and 6 h, at room conditions.
The nanocrystalline structure was determined on an x-ray diffraction (XRD) meter (SIEMENS D5000) using the Cu-Kα radiation at λ = 1.5406 Å. The mean grain sizes and grain size distributions of the nanosystems were determined by means of various techniques involving the use of a commercial WIN-CRYSIZE program packet based on the Warren–Averbach formalism to analyze the XRD data. The UV–Vis absorption spectra of TiO2 and V2O5/TiO2 samples were taken by CARRY 5000 UV–Vis–NIR. The Brunauer–Emmet–Teller (BET) specific surface area of the samples was determined by nitrogen adsorption/desorption at 77 K. The catalytic activity of the materials was studied by degradation of methylene blue under solar light.
3. Characterizations of material
3.1. Crystal structure, morphology, particle size and the BET specific surface area
Figure 1 shows XRD patterns of TiO2 sample before grinding (T0), TiO2 samples after grinding for 2, 4, 6 h (T2, T4 and T6) and V2O5/TiO2 sample ground for 4 h (VT). There are only specific peaks of TiO2 rutile and no peak of V2O5. The XRD patterns showed that samples synthesized are single phase—rutile phase. Peaks of sample T0 are higher and narrower than those of TiO2 particles T2, T4, T6 and VT. Thus, the particle size of TiO2 sample before grinding (T0) is larger than that of TiO2 ground samples (T2, T4, T6). This result is clear and suitable with a decrease in particle size when increasing grinding time. The average crystal sizes and the BET specific surface area values of these samples are given in table 1.
Figure 1. XRD patterns of TiO2 (T0, T2, T4, T6) and VT.
Download figure:
Standard image High-resolution imageTable 1. Crystal size and BET values of samples.
| Sample | Symbol | Grinding time (h) | Average crystal size (nm) | BET (m2 g−1) |
|---|---|---|---|---|
| TiO2 | T0 | 0 | 130 | 7.18 |
| T2 | 2 | 40 | 9.84 | |
| T4 | 4 | 14 | 15.12 | |
| T6 | 6 | 13 | 15.20 | |
| V2O5/TiO2 | VT | 4 | 22 | 20.80 |
From table 1 it is seen that the ground particle size decreased significantly as grinding time increased. However, when grinding time increased from 4 to 6 h, the TiO2 particle sizes and BET specific surface area nearly did not change. So the grinding time chosen to synthesis V2O5/TiO2 sample was 4 h. In the case of V2O5/TiO2, the BET specific surface area increased noticeably (20.8 m2 g−1) when compared with TiO2 which was also ground for 4 h (15.12 m2 g−1).
There is no peak of vanadium in XRD pattern of V2O5/TiO2 sample. This phenomenon was like that in [9]. There was also no peak of vanadium oxide in the XRD patterns but x-ray absorption spectroscopy (XAS) analysis indicating V4+ instead of V5+ implied that vanadium either substituted Ti4+ site or embedded in the vacancy of TiO2 structure. Therefore, either vanadium was incorporated in the crystalline of TiO2 or vanadium oxide was very small and highly dispersed.
3.2. UV–Vis absorption spectra and band gap energy calculation
To study the light absorption ability of materials, we determined UV–Vis absorption spectra of TiO2 and V2O5/TiO2 samples. The results are given in figure 2.
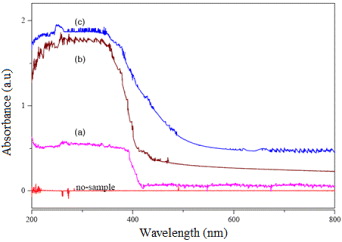
Figure 2. UV–Vis absorption spectra of TiO2: (a) T0, (b) T4, (c) V2O5/TiO2 (VT) nanoparticle and V2O5.
Download figure:
Standard image High-resolution imageFigure 2 shows that the sample T0 absorbs light having wavelength below 420 nm, while the samples T4 and VT absorb light at longer wavelengths and induce red-shift. The samples T4 and VT can absorb mostly at the region below 480 and 570 nm, respectively, with much higher absorption coefficients. These samples show even the absorption at the longer wavelength regions. The reason perhaps is the difference in particle size of the same sample. In the case of VT, the interaction between V2O5 and TiO2 is the explanation for its red-shift and for the increase in its absorption coefficient.
In figure 2 we can see that the UV–Vis absorption spectrum of V2O5/TiO2 (figure 2(c)) is really spectrum of a composite of TiO2 and V2O5 but not that of separated TiO2 (figure 2(b)) or V2O5 (we can find the absorption spectrum of V2O5 in [9]). This evidence demonstrates the interaction between V2O5 and TiO2 after grinding and V2O5 may incorporate into the lattice of TiO2 rutile.
On the other hand, as we know, the bulk TiO2 crystal is an indirect semiconductor, its absorption index α(hν) should closely correlate with the behavior of the form (hν − Eg)3/2. Normally nanomaterials exhibit an absorption spectrum such as that of the direct band gap semiconductor, and its absorption coefficient α(hν) follows the (hν − Eg)1/2 law [14]. Based on the absorption data (from 400 to 800 nm) presented in figure 2 we plot the curves of (αhν)2 in dependence on energy hν in figure 3.
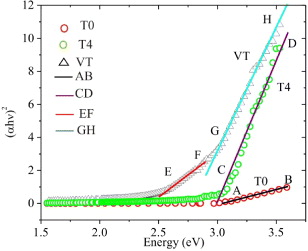
Figure 3. Curves of (αhν)2 versus hν for T0, T4 and VT samples.
Download figure:
Standard image High-resolution imageIt is shown that the T0 and T4 curves contain AB and CD linear lines, respectively. The prolongations of these lines cut the x-axis at 3.04 and 2.99 eV, respectively. These energy values approximate to the band gap energy value of TiO2 rutile (3.0 eV). Similarly, the VT curve includes the EF and GH linear lines that the x-axis intersects at 2.43 and 2.79 eV, respectively. The former value 2.43 eV is close to the band gap energy value of V2O5. The latter is considered the band gap energy value of TiO2 doped by vanadium. This value is between the band gap energy values of TiO2 rutile and V2O5. Doping vanadium could result in the 3d orbital of vanadium changing the band gap of titania. Thus, the optical absorption edge of TiO2 shifts to visible light and the band gap of vanadium-doped TiO2 is narrower than that of TiO2 [15].
4. Photocatalytic activity of the V2O5/TiO2 material
As above, V2O5/TiO2 material can absorb the light not only in the ultraviolet region but also in the visible region. In order to determine photocatalytic activity of the V2O5/TiO2 material, we carried out degradation of methylene blue on VT under solar light.
A weight 0.1 g of catalyst powder was suspended in 20 ml of 5.10−5 M methylene blue (MB) aqueous solution and the mixture was stirred under solar light at room temperature. The concentration changes of MB were determined by the UV–Vis spectrometer Jasco 630.
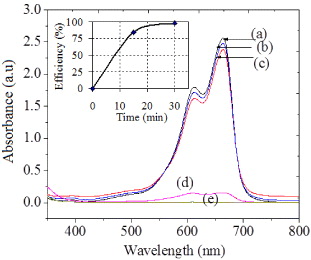
Figure 4 shows the absorption spectra of MB with concentration 5.10−5 M (a), of the degraded MB by T0 (b), T4 (c) and VT after 15 min (d) and by VT after 30 min (e). Whereas TiO2 material with large particle size mostly shows inactivity in reaction with methylene blue under solar light, the TiO2 milled in 4 h has smaller size and exhibits some more photocatalytic activity. The degradation of methylene blue on VT was very fast and most of the methylene blue was degraded after 30 min (curve (e) in figure 4). So, the obtained V2O5/TiO2 material has high photocatalytic activity in degradation of methylene blue due to the good combination of V2O5 and TiO2 in the nano crystalline lattice. These results open the way for a new application in environmental treatment.
Figure 4. UV–Vis spectra of methylene blue 5.10−4 M (a), degraded by T0 (b), by T4 (c) and by VT after 15 min (d), after 30 min (e).
Download figure:
Standard image High-resolution imageThe photocatalytic activity of present vanadium-doped TiO2 rutile could be compared to that of some TiO2 anatase and TiO2 rutile which had been published previously by the author groups of Anpo et al [8] and Liu et al [12], respectively.
5. Conclusions
The grinding reactive method exhibits successful technique to synthesize vanadium-doped titania rutile TiO2. By this route, we can obtain nano composite particles of metal-doped TiO2 rutile without controlling the calcination procedure or using expensive starting chemicals.
The vanadium-doped titania rutile shows a red-shift in the UV–Vis spectra due to the small size of nano rutile particles (with BG more than that of anatase TiO2) and promotion of vanadium.
The results of methylene blue photocatalytic degradation indicate that the nano vanadium-doped TiO2 rutile obtained has remarkable higher activity than that of pure nano TiO2 rutile under solar light at room temperature.
Acknowledgments
This work is partially financially supported by Vietnam Academy of Science and Technology. The authors are thankful to Professor Acad. Nguyen Van Hieu for his help and encouragement.