Abstract
Phyllanthus amarus (P. amarus) is commonly used for traditional Indian medicine and as dietary adjuncts for the treatment of numerous physiological disorders including hepatic disorders. Due to the poor water solubility of its major constituents such as lignans and flavonoids, its absorption upon oral administration could be limited. The present study was designed to evaluate and compare the hepatoprotective effects of the ethanolic extract of P. amarus (PAE) and its nanoparticles (PAN) on paracetamol induced acute liver toxicity in Sprague–Dawley rats. An oral dose of PAE at 125 and 250 mg kg−1 and PAN at 25 and 50 mg kg−1 showed a significant hepatoprotective effect relatively to the same extent (P < 0.001) by reducing levels of aspartate aminotransferase, alanine aminotransferase, alkaline phosphatase and bile salts. These biochemical assessments were supported by rat hepatic biopsy examinations. Moreover, the results also indicated that the hepatoprotective effect of 50 mg kg−1 PAN was effectively better than 125 mg kg−1 PAE (P < 0.001), and an oral dose of PAN that is five times less than PAE could exhibit similar levels of outcomes. In conclusion, we suggest that the nanoparticles system can be applied to overcome other poorly water soluble herbal medicines and furthermore to decrease the treatment dosage.
Export citation and abstract BibTeX RIS

Content from this work may be used under the terms of the Creative Commons Attribution 3.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Hepatic disorder remains a serious health problem, and is caused by chemicals, drugs and alcohol. The hepatic system plays a crucial role in metabolism of drugs and xenobiotics, and in maintaining biological equilibrium. In spite of the remarkable strides in modern medicine, there is hardly any drug that potentiates liver function, offers protection to the hepatic cells from damage or helps regeneration of hepatic cells [1]. Herbal drugs are frequently considered to be less toxic and free from side-effects than synthetic drugs. The search for bioactive compounds of plant origin with potent hepatoprotective activity has become a central focus for study of hepatoprotection today.
Focusing our attention on natural, nontoxic and bioavailable sources of hepatoprotectants, we undertook to investigate the hepatoprotective activity of Phyllantus amarus (P. amarus) Schum. & Thonn. (Euphorbiaceae). It contains many types of phenolic compounds viz. lignans such as phyllanthin and hypophyllanthin; flavonoids such as quercetin and astragalin; ellagitannins such as amarinic acid, amarin and phyllanthisiin D [2–7]. These phenolic compounds are related to antioxidant activity [8]. Moreover, water/alcohol extract of P. amarus blocks HIV-1 attachment and the HIV-1 enzymes integrase, reverse transcriptase and protease to different degrees [9]. Furthermore, it is proven for its potent hepatoprotection against carbon tetrachloride induced hepatic injury [10]. Besides this, phyllanthin and hypophyllanthin were reported to reduce hepatotoxicity induced by carbon tetrachloride and galactosamine in rats and may be used as marker for hepatoprotection of P. amarus [7,11]. Since these constituents belong to the group of compounds with poor water solubility, their absorption upon oral administration could be limited. Therefore, an attempt has been made to enhance the dissolution rates and hence bioavailability of the P. amarus extract by preparing its nanoparticles.
Nanotechnology is an attractive area of research related to production of nanoparticles of variable size and shape, as well as their possible benefits in clinical medicines [12–14]. The rapid development of nanotechnology has opened the possibility of controlling and manipulating structures at the molecular level and led to the creation of novel surface architectures and materials. Traditional biomedical applications incorporate the use of nanotechnology in a broad spectrum of areas. Among them, biosensors, tissues engineering, intelligent systems and nanocomposites are used in implant design and controlled release systems [15]. Several nano-oriented approaches are being intended in order to optimize the technological aspects of drugs. The use of these processes has dramatically enhanced dissolution rates in vitro and bioavailabilities in vivo of many drugs [16].
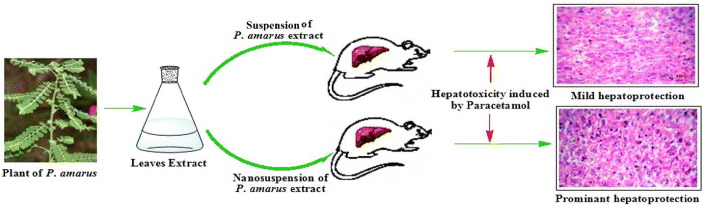
In the present study, our aim was to evaluate and compare the hepatoprotective effects of the ethanolic extract of P. amarus (PAE) and of its nanoparticles (PANs) on paracetamol induced acute liver injury in rats (figure 1). The hepatoprotective effects of the PAE and PANs were determined by assessing levels of total bilirubin (mg%), direct bilirubin (mg%), alanine aminotransferase (ALT), alkaline phosphatase (ALP), aspartate aminotransferase (AST) and also by examining the histopathological sections of the liver, all of which are associated with hepatic integrity.
Figure 1. Schematic representation of hepatoprotective effect of ethanolic extract of P. amarus and its nanoparticles on paracetamol induced acute liver toxicity in rats.
Download figure:
Standard image High-resolution image2. Materials and methods
2.1. Materials and chemicals
The plant P. amarus Schum. & Thonn. was obtained and taxonomically identified by Botanical Survey of India, Allahabad. A voucher specimen no. AC-593/11 was deposited in the department for future reference. Technical grade paracetamol (purity 99.4%) was obtained from Merck, India and silymarin was obtained from Sigma Chemicals, USA. Bilirubin, ALP, ALT and AST were assayed by using kits from Ranbaxy diagnostic, New Delhi. All other reagents used were of analytical grade and obtained from Qualigens Fine Chemicals, Mumbai, India.
2.2. Preparation of extract of P. amarus
The freshly collected leaves of P. amarus were dried in air followed by tray drier under control conditions and powdered. The powdered leaves (1000 g) were macerated with petroleum ether to remove fatty substances; the marc was further exhaustively extracted with 95% ethanol for 3 d (3 × 3 l) by cold percolation method and centrifugation at 10 000 rev min−1. The extract was separated by filtration and concentrated on rotavapour (Buchi, USA) and then dried in lyophilizer (Labconco, USA) under reduced pressure and thus 120.0 g of solid residue (yield 12% w/w) was obtained. The extract was stored in a desiccator for use in the subsequent experiment.
2.3. Preparation of nanoparticles of P. amarus extract
Nano precipitation technique was applied to prepare PANs with slight modification of a previously reported process [17]. Briefly, 5 g of PAE was dissolved in 30 ml of acetone and ethanol (3:1) by sonication at 20 W for 30 s. The resulting solution was then slowly injected (1 ml min−1) with a syringe connected to a thin teflon tube, into 50 ml water containing polyvinyl alcohol (PVA) 1.5% w/v with continuous magnetic stirring at 1000 rpm. The resulting emulsion obtained was then diluted in 100 ml PVA solution (0.2% w/v in water) in order to minimize coalescence and the mixture was continuously stirred (500 rpm) for 6 h at room temperature to allow solvent evaporation and nanoparticle formation. The resulting nanosuspension was subsequently cooled down to −18 °C and freeze dried.
2.4. Particle size analysis and morphological characterization
Mean particle size and size distribution (polydispersity index) were determined using photon correlation spectroscopy (Zetasizer, Beckman Coulter Inc., Delsa Nano 4C). The size distribution analysis was performed at a scattering angle of 90° and at a temperature of 25 °C using samples appropriately diluted with filtered water (0.2 μm filter, Minisart, Germany). All measurements were performed in triplicate (n = 3) and the standard deviation (SD) was recorded. In addition, the morphological characterization was performed using transmission electron microscope (TEM). TEM images were collected using Technai 30G2 S-Twin electron microscope. Sample of the nanoparticles suspension (5–10 μl) were dropped onto Formvar-coated copper grids. After complete drying, the samples were stained using 2 %w/v phosphotungstic acid. Digital Micrograph and Soft Imaging Viewer software were used to perform the image capture and analysis, including particle sizing.
2.5. Test animals
Sprague–Dawley rats weighing between 150 and 200 g and free of any signs of gross abnormalities were used for in vivo studies. The rats were acclimatized in our animal facilities for 1 week before study. The animals were housed at 22 °C ± 2 °C temperature with 12 h light/dark cycle and 50–70% relative humidity. The rats were individually housed with food and water provided ad libitum. The various in vivo studies were performed in accordance with the Institutional Animal Ethics Committee constituted as per directions of the Committee for the Purpose of Control and Supervision of Experiments on Animals under the Ministry of Animal Welfare Division, Government of India, New Delhi.
2.6. Paracetamol-induced hepatotoxicity in rats
Hepatotoxicity was induced by paracetamol with slight modification of that of a previously reported method [18]. Rats were randomly divided into seven groups of six rats each. Groups I and II were given vehicle gum acacia (5 mg kg−1 p.o). Groups III and IV were treated with two doses (125 and 250 mg kg−1 b.w. p.o.) of ethanolic extract in 5%w/w acacia suspension, the standard drug silymarin 25 mg kg−1 p.o. was given to group V. Groups VI and VII were treated with PANs (25 and 50 mg kg−1, p.o.), respectively, for 7 d. On the seventh day paracetamol suspension was administered at a dose of 750 mg kg−1 b.w. i.p. to the animals of all groups except group I. The blood was collected by cardiac puncture in heparinized tubes. The liver was immediately taken out and washed with ice-cold saline, then weighed and stored at −80 °C. The blood and liver samples were assessed for their biochemical and histopathological investigation.
2.7. Biochemical estimation
Collected blood was centrifuged at 3000 rpm at 4 °C for 10 min to obtain the plasma serum which was analyzed for different biochemical parameters such as total bilirubin, direct bilirubin, AST, ALT and ALP. For histopathological study, liver tissue was quickly removed after autopsy and fixed in 10% formosaline.
2.8. Statistical analysis
Results were analyzed statistically by one-way ANOVA followed by Dunnette multiple comparisons test using graph prism software. The minimum level of significance was set at p < 0.001.
3. Results
3.1. Particle size analysis and morphological characterization
The mean particle size and polydispersity index (PI) were measured immediately after the preparation of the nanosuspension. The mean particle size and PI for PANs were found to be 243 ± 9.7 and 0.269 ± 0.053 nm, respectively. The PI is a measure of particles size distribution. Its value less than 0.3, indicates a high degree of homogeneity in particle size and vice versa.
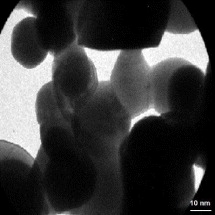
Figure 2 reports the TEM micro photomicrograph of the PANs. The picture reveals an irregular and asymmetrical morphology and solid dense structure, and processing the TEM image, the size of the particles resulted between 205 and 310 nm, according to the photon correlation spectroscopy (PCS) analysis.
Figure 2. TEM image of PANs.
Download figure:
Standard image High-resolution image3.2. Effect on biochemical markers in PCM induced hepatotoxicity
A marked increase in total bilirubin levels from 0.201 mg dl−1 in control rats (group I) to 0.814 mg dl−1 in paracetamol (PCM) induced rats (group II) was observed. However, the total bilirubin levels were reduced to 0.521 and 0.317 mg dl−1 with the treatment of 125 mg kg−1 (group III) and 250 mg kg−1 (group IV) of PAE, respectively. Interestingly, the comparatively lower doses i.e. 25 mg kg−1 (group VI) and 50 mg kg−1 (group VII) of PANs have effectively restored the total bilirubin levels to 0.525 mg dl−1 and 0.323 mg dl−1, respectively. In addition, the standard silymarin (25 mg kg−1), a hepatoprotective drug, has restored the total bilirubin levels significantly i.e. 0.284 mg dl−1.
Serum AST (International unit l−1 or IU l−1) levels were also elevated from 85.04 IU l−1 in the control group to 133.42 IU l−1 in the PCM treated group. Treatment with standard silymarin 25 mg kg−1 has brought back the AST to the near normal levels i.e. 89.00 IU l−1. However, treatment with the 125 and 250 mg kg−1 of PAE restored the AST levels up to 105.48 and 93.24 IU l−1, respectively. Furthermore, the AST levels were reversed significantly up to 109 and 94.27 IU l−1 with the treatment of 25 mg kg−1 and 50 mg/kg PANs, respectively.
In the case of ALT, there was a noticeable rise from 29.12 IU l−1 in control rats to 81.28 IU l−1 in PCM induced rats was observed. Silymarin (25 mg kg−1) decreased levels of serum ALT (47 IU l−1) in the induced rats. In addition, the PAE significantly reduced levels of persisting ALT activity up to 55.84 and 36.24 IU l−1 with the dose of 125 and 250 mg kg−1, respectively. Furthermore, the comparatively lower doses i.e. 25 and 50 mg kg−1 of PANs have effectively restored the ALT levels to 57.43 IU l−1 and 36.91 IU l-1, respectively.
Considerable increase in the serum ALP levels were observed in PCM induced rats i.e. 221.20 IU l−1 compared to 87.10 IU l−1 in control rats. However, the serum ALP levels were reduced to 135.96 and 96.42 IU l−1 with the treatment of 125 and 250 mg kg−1 of PAE, respectively. Interestingly, the comparatively lower doses i.e. 25 and 50 mg kg−1 of PAN have significantly restored the serum ALP levels to 137.18 and 97.56 IU l−1, respectively. In addition, the silymarin (25 mg kg−1) has restored the serum ALP levels significantly i.e. 93.22 IU l−1.
3.3. Histopathological observation of liver
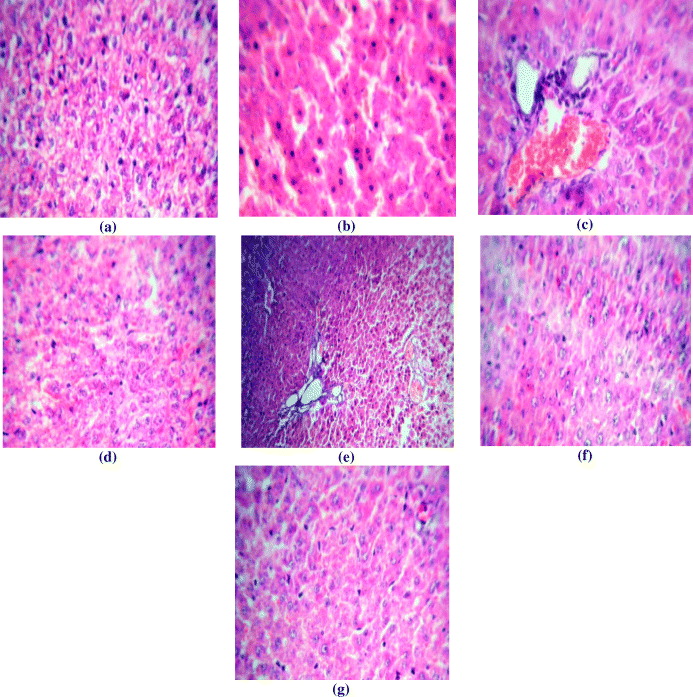
In normal rats (figure 3(a)), the architecture is normal. The central veins, sinusoids and portal triads appear normal. The hepatocytes show moderate cytoplasm and round to oval nuclei. There is no periportal inflammation. In paracetamol induced rats (figure 3(b)) the central veins show dilatation and congestion. The hepatocytes show feathery degeneration. The portal triads show mild peri-portal inflammation composed of lymphocytes. In the ethanolic extract (125 mg kg−1) treated group (figure 3(c)) the hepatocytes show moderate cytoplasm and moderately enlarged pleomorphic and hyperchromatic nuclei. The portal triads show mild peri-portal inflammation composed of lymphocytes. The central veins are normal. Ethanolic extract (250 mg kg−1) treated rats (figure 3(d)) shows that the architecture is normal. The central veins, sinusoids and portal triads appear normal. The hepatocytes show moderate cytoplasm and round to oval nuclei. In the silymarin treated group (figure 3(e)) the central veins appear normal. The hepatocytes show feathery degeneration. The portal triads show mild peri-portal inflammation composed of lymphocytes. PAN (25 mg) treated rats (figure 3(f)) the section shows large dilated and congested central veins. The hepatocytes are normal. The portal triads appear normal whereas PAN (50 mg) treated rat (figure 3(g)) shows normal architecture. The central veins, sinusoids and portal triads appear normal. The hepatocytes show moderate cytoplasm and round to oval nuclei. There is no peri-portal inflammation.
Figure 3. Histopathology of liver: (a) Group I , (b) group II, (c) group III, (d) group VI, (e) group V, (f) group VI and (g) group VII.
Download figure:
Standard image High-resolution image4. Discussion
This study was carried out to compare the hepatoprotective activity of PAE and PANs on PCM induced acute hepatic injury in rat. The PAE nanoparticles extract was successfully prepared by nanoprecipitation method and developed into stable nanosuspension. This novel attempt was taken to enhance the bioavailability and reduce the dose of herbal medicine with poor water solubility. Previous studies have demonstrated that the same concentration of nanoparticle formulation possesses better pharmacological activity as compared with its raw drug [19].
Nanosuspensions of drugs are sub-micron colloidal dispersions of pure particles of drug, which are stabilized by surfactants and can be used for compounds that are poorly water soluble [20]. By decreasing the particle size of the solid form of the drug, the dissolution rate is increased, thereby addressing a number of issues related to poor oral bioavailability. The solid state of the drug offers solutions to issues of chemical stability, and the small size confers increased physical stability with respect to sedimentation [21].
Moreover, nanonization confers an additional advantage of higher mass per volume loading. This is crucial when high dosing is required. Conventional approaches often attempt to solubilize insoluble drugs with the use of excessive amounts of cosolvents, but this poses toxicity problems. The above-described advantages have driven the development of nanosuspension technology, which has subsequently revealed secondary benefits that are now beginning to be realized [22]. The mean particle size for PANs was found to be 243 ± 9.7 nm. It depends on the nature of solvent and non-solvent used for preparation of nanoparticles through nanoprecipitation technique [23]. The interest of using alcohol and acetone as a solvents essentially lies in their relatively low dielectric constant (ε value). Indeed, the lower the dielectric constant value, the more the solvent will dissolve the hydrophobic compounds. Water was used as a non-solvent due to its comparatively high dielectric constant value (80.1); this makes it a better dispersing solvent for poorly water soluble compounds. Moreover, the alcohol and acetone are not a concern in terms of toxicity. Indeed, they belong to class 3 according to the International Conference on Harmonization solvent toxicity scale (class 3 solvents present very low risks to human health).
Hepatotoxicity induced by PCM is the most commonly used model system for the screening of hepatoprotective activity of plant extracts and drugs [24,25]. In this study, a significant increase in the level of total bilirubin, direct bilirubin, AST, ALT and ALP in the serum was observed after administration of PCM, indicating chemical induced hepatocellular toxicity. Serum levels of bile salt and hepatic enzymes are very sensitive markers employed in the diagnosis of liver diseases [26]. When the hepatocellular plasma membrane is damaged, the enzymes normally present in the cytosol are released into the blood stream. This can be quantified to assess the type and extent of hepatocellular damage. ALP is excreted normally via bile by the liver. The liver injury due to toxins can result in defective excretion of bile by hepatocytes which are reflected as their increased levels in serum [27]. However, the increased levels of these markers were significantly decreased by pretreatment with PAE, implying that PAE may stabilize the hepatic cellular membrane and protect the hepatocytes against toxic effects of PCM, which may decrease the rate of leakage of the enzymes into the bloodstream [28].
In this study, a significant increase in the serum enzyme levels, namely AST, ALT, ALP and bile salt was observed in rats receiving PCM in the vehicle (group II) when compared to normal (group I) rats administered with the vehicle alone. However, the level of these serum enzymes and bile salt were significantly (P < 0.001) lower in rats treated with the PAE (groups III and IV) than in group II rats. The protection offered by silymarin (group V) was found to be higher. In addition, the comparatively lower doses (25 and 50 mg) of PAN similarly ameliorated the hepatocellular damage (groups VI and VII) from the PCM-induced hepatotoxicity in rats with nearly the same effectiveness as that observed with the PAE. The reason for elevated hepatoprotective activity of PANs may be due to increase in bioavailability of the PANs. The nanonization of P. amarus results in the production of numerous nanoparticles with increased surface area, due to which the dissolution rate of the nanoparticles becomes amplified and hence its absorption in vivo increases.
Furthermore, the above findings are confirmed by the histopathology studies of the liver tissue. When compared to the histoarchitechture of the livers of group I (control) rats (figure 3(a)), liver cells of group II (exposed to PCM) reveal extensive damage, characterized by the disruption of the integrity of hepatocyte, damaged cell membranes, degenerated nuclei, a disintegrated central vein and damaged hepatic sinusoids (figure 3(b)). In groups III and IV, rats (exposed to PCM + PAE) had improved histopathological changes as compared to the PCM intoxicated group without treatment (figures 3(c) and 3(d)). In addition, the PANs similarly ameliorated the histopathological damage from the PCM induced hepatotoxicity in rats with nearly the same effectiveness as found with PAE.
In conclusion, we found that an oral dose of PANs that is five times less than the oral dose of PAE could exhibit a similar hepatoprotective effect. Thus, we suggest that the nanoparticles system can be applied to overcome other water poorly soluble herbal medicines and furthermore to decrease the treatment dosage. This study could serve as a useful reference to allow the future exploitation of nanoparticulate system as a novel preventive and therapeutic measure for the treatment of hepatic and other various physiological disorders.
Acknowledgments
ACP acknowledges funding support from the Department of Science and Technology (DST), Goverment of India and FA 5209–11-P-0160, ITC-PAC fund support.