Abstract
Nanostructured composite electrodes of MnO2/C with various types of carbons (noir, mesoporous carbon and carbon vulcan) were prepared by chemical reduction. The combination of carbon vulcan and carbon nanotubes (CNTs) was also studied at the total content of 30 wt%, etc. The morphology and structure of samples were examined by scanning electron microscopy (SEM) and x-ray diffraction (XRD). The specific capacitances of the composite electrodes were determined by cyclic voltammetry measured in 2M NaCl at different potential scan rates. The carbon addition improves specific capacitance (SC) as well as specific surface area (SSA). Compared to other carbon, the highest SC value was reached at 30% content for carbon vulcan. The addition of 5 wt% CNTs in MnO2/carbon vulcan increased the SC up to 199 F g−1 and improved significantly the cycling behavior of composite electrode.
Export citation and abstract BibTeX RIS

Content from this work may be used under the terms of the Creative Commons Attribution 3.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
In recent years, nanomaterials have been of key importance for future electrochemical energy storage and conversion devices, such as the supercapacitor, lithium-ion battery and fuel cell. The electrochemical power sources substituting fossil fuels can limit the effect of climate change on Earth [1–3]. Nanostructured materials and nanotechnologies have attracted great interest due to the unusual properties endowed (electronic, electrochemistry, mechanic, optical and surface area, etc) [4].
As alternative energy storage devices, electrochemical capacitors and ultracapacitors are promising power sources for automobile application and the portable electronics market [3, 5]. Although supercapacitors possess high power density and long life cycle, their negative aspects are low energy density and high production cost [3, 6]. The best way to solve these problems is to develop new electrode materials, designing nanostructured electrode configuration, etc [7, 8]. Manganese dioxide is considered a promising candidate for electrode material in supercapacitors because of their low cost, abundance and environmentally friendly nature [9]. However, these materials exhibit the inherent disadvantages of low conductivity and low specific capacitance [9]. Thus, the improvement pursued in manganese oxide properties mainly concerns electronic conductivity, specific capacitance, charge–discharge capability and durability [10, 11]. By using the nanostructured electrode configuration, we can overcome the challenges of electronic conductivity, charge–discharge capacitance and cycling behavior.
In this work, we focus on synthesis condition, electrode configuration design, morphology characterization and electrochemical properties of nanostructured composite electrodes MnO2/C (noir, mesoporous carbon, carbon vulcan) with 30 wt% carbon content [12]. Nanostructure carbon, CNTs, combined with carbon vulcan at 2.5, 5 and 7.5% was also studied.
2. Experimental
2.1. Synthesis of MnO2/C electrode materials
Chemical manganese dioxide (CMD) was synthesized by reaction between KMnO4 (Merck) and MnSO4 (Sigma Aldrich, >99%). The carbon powder was first added in deionized water and kept stirring for 20 min to obtain a homogenous suspension. Then KMnO4 and MnSO4 with a molar ratio of 3 : 2 were continuously added into the suspension and all the mixture was stirred for 1 h at 80 °C. The CMD product was finally washed many times by distilled water and dried at 120 °C for 48 h. Here we study the effect of carbon types on structure, morphology and electrochemical properties of composite electrodes. Mesoporous carbon black (MC), carbon vulcan-XC72R (VC), noir and CNTs were purchased from Sigma-Aldrich, Carbot and PINACO-Vietnam.
2.2. Structure and morphology characterization
Samples were analyzed by powder x-ray diffraction (XRD) using a PANalytical X'Pert MPD diffractometer with CuKα radiation, 0.05° step and 8 s per step counting time to minimize noise. The morphology and the distribution of grain size were determined using a field emission scanning electron microscopy (FE-SEM) ZEISS ULTRA 55 instrument equipped with energy dispersive x-ray (EDX) spectroscopy analysis.
2.3. Formulation of composite electrode and electrochemical measurements
The composite electrode was prepared by dipping graphite substrate in the mixture of MnO2/C powder, isopropanol and PTFE with a weight ratio MnO2/C: isopropanol: PTFE = 65:30:5. The electrode was dried at room temperature for 24 h and then at 120 °C for 1 h.
All electrochemical measurements of materials were performed in a three-electrode cell including MnO2/C electrode, platinum mesh and Ag/AgCl (0.198 V) (Biologic) as working, counter and reference electrode. The cyclic voltammetry (CV) measurement was carried out on an Autolab PGSTAT302N Potentiostat/Galvanostat in 2 M NaCl from 0 to 1 V at a scan rate 50 m V s−1.
3. Results and discussion
3.1. Structural and morphology characterization
Figure 1 illustrates the XRD patterns of MnO2/C composite electrodes with different types of carbon: carbon vulcan, mesoporous carbon and carbon vulcan/CNTs at 30 wt% content. The diffraction peaks at 2θ (CuKα) = 37.2°, 42.7°, 58°, 78°, 90° correspond to the structure of epsilon manganese dioxide (ε-MnO2), which is a defective structure of γ-MnO2 [13]. The MnO2/carbon vulcan composite electrode with 5% CNTs addition exhibits the same structure (figure 1(b)). Thus, the MnO2/C structure is not affected by carbon content and types of carbon. However, the synthesis conditions such as solution concentration and temperature can influence the structure of electrode materials [14, 15].
Figure 1. (a) XRD patterns of MnO2/C electrodes with various carbons; (b) XRD patterns of MnO2/C with 5% CNTs added.
Download figure:
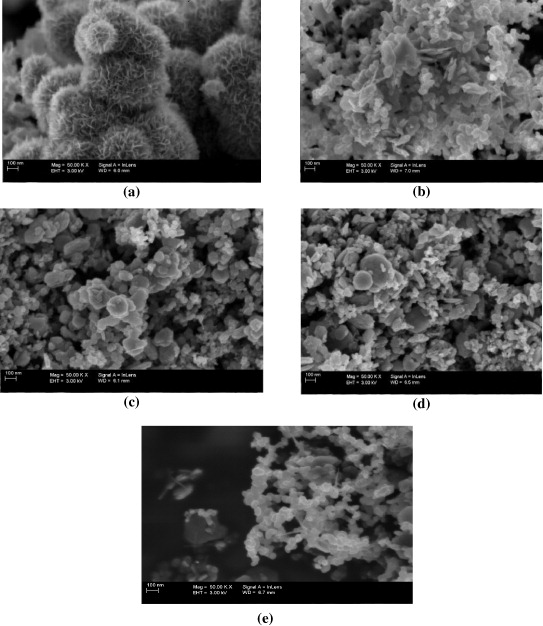
Standard image High-resolution imageAs shown in figure 2, the morphology of CMD based composite electrodes was analyzed by SEM images in changing types of carbon. MnO2 sample prepared by chemical reaction exhibited nanostructure with mono-dispersed flower-like particles (figure 2(a)). With all MnO2/C samples, the particles have random shapes when using carbon noir, mesoporous carbon or carbon vulcan as seen in figures 2(b), (c) and (d). In addition, a wide distribution of particle sizes was also obtained. However, the addition of 5 wt% CNTs for MnO2/carbon vulcan gives the homogenous and fine particles of diameters about 50–70nm (figure 2(e)).
Figure 2. (a) SEM images of MnO2; (b, c, d) MnO2/C electrodes with various type of carbons; (e) MnO2/carbon vulcan with 5% CNTs added.
Download figure:
Standard image High-resolution image3.2. Electrochemical stability
The electrochemical properties of MnO2/C samples were characterized by cyclic voltammetry in 2.0 M NaCl solution in the potential window of 0–1 V versus Ag/AgCl at different scan rates. The specific capacitance (SC) of MnO2/C was calculated by the following equation [15]:

where SC is the specific capacitance, Q is charge (C), ΔE is the voltage window (1V) and m is the active material mass (g).
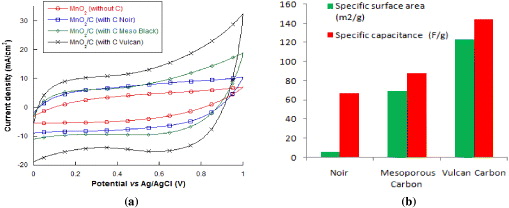
Figure 3(a) shows the CV curves of CMD based composite electrodes in 2.0 M NaCl solution at a scan rate 50 mV s−1. Without the carbon additional, the SC of MnO2 active material was 35 F g−1. At 30 wt% content, the SC of MnO2/C electrodes are 67, 88 and 144 F g−1 for carbon Noir, mesoporous carbon and carbon vulcan, respectively. Indeed, in using various types of carbon, the SC of MnO2/C changes due to the variation of specific surface area (figure 3(b)). It could be explained that the carbon performs not only as a conductive agent but also as the template supporting dispersion of MnO2 nanoparticles and stabilization of MnO2 structure [10, 12, 13].
Figure 3. (a) CV curves of MnO2, MnO2/C with various types of carbon, (b) relationship between specific surface area and specific capacitance of MnO2/C with various types of carbons.
Download figure:
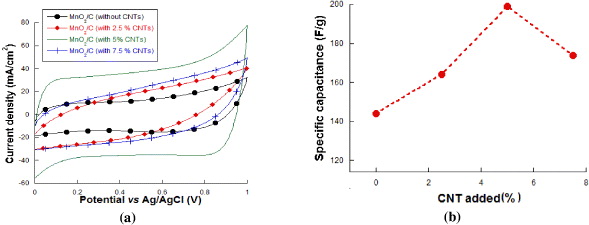
Standard image High-resolution imageFigure 4(a) shows the CVs of MnO2/carbon vulcan-CNTs based composite electrodes in 2.0 M NaCl solution at a scan rate 50 mV s−1 when CNTs addition changes from 0 to 2.5, 5 and 7.5%. When CNTs content increases, the SC of MnO2/C increases and reaches the highest value at 5 wt% CNTs (199 F g−1). When the addition of CNTs varies from 5 to 7.5%, the SC of MnO2/C electrode does not change (figure 4(b)). Thus, the presence of CNTs increases the SC due to stability of defective structure and improvement of charge transfer between material and electrolyte [16].
Figure 4. CV curves of MnO2/C at different ratios of CNT added, (b) relationship between % CNT added and specific capacitance of MnO2/C.
Download figure:
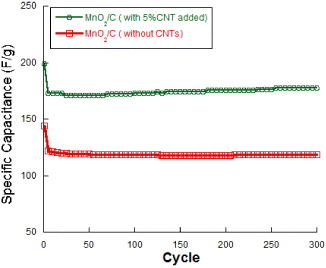
Standard image High-resolution imageFigure 5 shows the charge–discharge capacitance at different cycles calculated from CV curves. It can be seen that MnO2/C composite electrodes exhibit high electrochemical reversibility and cycling stability after 300 cycles. With MnO2/carbon vulcan, the SC gradually reduced and lost nearly 20% initial SC after 300 cycles. In contrast, the specific capacitance of MnO2/carbon vulcan-CNTs (5%) showed excellent cycling performance and lost only 10% after 300 cycles. This behavior can be explained by good ionic conductivity network between MnO2 particles and conducting additives using CNTs. Indeed, CNTs facilitate fast electron transport on the electrode surface and develop good electrolyte–electrode interface due to the high active surface area of homogenous nanometric particles [17, 18].
Figure 5. Specific capacitance of MnO2/C without CNTs and with 5% CNTs added calculated from 300 charge–discharge cycle at a scan rate 50 mV s−1.
Download figure:
Standard image High-resolution image4. Conclusion
The MnO2/C composite electrodes were prepared by chemical reduction, using various types of carbon and within addition of CNTs. All electrode materials show ε-MnO2 structure (defect of γ-MnO2). By adding CNTs, the MnO2/carbon vulcan-CNT electrodes give particularly homogenous nanoparticles size about 50–70 nm. Furthermore, these electrodes improve the SC and also the cycling behavior, especially at 5 wt% CNTs addition.
Acknowledgments
This work was supported by Vietnam National University in Ho Chi Minh City through the Science and Technology Fund granted for the B2011-18–01 TĐ.