Abstract
Recently carbon nanotubes (CNTs)-reinforced metal matrix composites (MMCs) have attracted increasing attention due to their promising properties. Most research on metallic matrix–CNTs composites (MMCs–CNTs) show that uniform dispersion of CNTs has been by far the most significant challenge in the field of CNTs-reinforced composites. In this research we will present an approach to obtain homogeneously dispersed CNTs in Al powders for preparing Al/CNTs nanocomposite. A novel polyester binder-assisted (PBA) mixing method was used for achieving uniform dispersion of CNTs, and power metallurgy (PM) technique was used for preparing Al/CNTs nanocomposite. The distribution quality of CNTs in Al matrix nanocomposites was also qualified based on image analysis technique. The morphologies, structures and mechanical properties of the Al/CNTs nanocomposite were also investigated in detail by scanning electron microscopy (SEM), energy dispersive x-ray (EDX) spectroscopy, x-ray diffraction (XRD) and mechanical measurement methods. Experimental results show that this method not only achieves good dispersion but it also avoids the damage on structure of CNTs by conventional mixing methods.
Export citation and abstract BibTeX RIS

Content from this work may be used under the terms of the Creative Commons Attribution 3.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Because of their excellent properties such as high elastic modulus, high strength and good thermal/electrical properties, carbon nanotubes (CNTs) have been attracting extensive interest [1–9]. One of CNTs' potential applications is in the use as reinforcement materials for metallic matrix composites to overcome the performance shortages of conventional materials [10–12]. Many recently published researchers have indicated that CNTs are ideal reinforcements to improve the mechanical properties of matrix due to their unique properties [13–16]. Besides, most recent research on metallic matrix–CNTs composites (MMCs–CNTs) has shown that the uniform dispersion of CNTs has been becoming the most significant challenge in the field of CNT-reinforced composites [17–19]. Several processing methods have been developed to improve CNT dispersion as well as the development of the new method to quantify carbon nanotube distribution and property correlation in nanocomposites [20–25]. These methods showed some promise, but all of them have limitations, such as nanoscale dispersion leading to good dispersion of CNTs only on the particle surface and depending on the metal particle size, ball milling leads to moderate or very good dispersion, but causes possible damage to CNTs and the molecular-level mixing method may lead to oxide impurities due to incomplete reduction of the powders [26]. To improve the homogeneous dispersion of CNTs in MMCs, innovation of method is indispensable.
This work studies the CNT dispersion condition in aluminum powders and investigates some of the properties of Al/CNTs nanocomposites. In this research, polyvinyl alcohol (PVA) was used as PBA material for researching the uniform dispersion of CNTs in Al matrix. Al/CNTs nanocomposite was produced by PM method and its properties were also investigated in detail in this research.
2. Experimental procedures
2.1. Materials
The commercially pure Al powder (purity >99.5%) with a size of 8–5 μm in diameter produced by Quanzhou Manfong Metal Powder Co., Ltd, was used for the matrix material. Multi-walled carbon nanotubes (provided by Institute of Materials Science, VAST) which were produced by using the thermal chemical vapor deposition method with a purity >95% and average diameter of about 10 nm and length 50 μm were used as reinforcement component. Polyvinyl alcohol (PVA), with a molecular weight of about 77 000 g mol−1, supplied by Perry Chemical Corp., China, was used as PBA agent.
2.2. Nanocomposite fabrication
Multi-walled carbon nanotubes were functionalized with carboxyl (–COOH) by treatment in the mixture of hot acid (HNO3:H2SO4, 1:3) at 60 °C in 6 h, then dried in argon atmosphere at 80 °C for 24 h. To improve the dispersive capacity of CNTs in Al matrix, MWCNTs with carboxyl function group were dispersed in ethanol by ultrasonic vibration to obtain a CNTs suspension. After that, this suspension was mixed with Al powders modified by PVA to form Al/CNTs nanocomposite powders. To remove PVA component, the Al/CNTs nanocomposite powder was heated in a flowing argon atmosphere at 400 °C for 2 h. Finally, the Al/CNTs nanocomposite powder was pressed in steel dies under a compress force of 2.5 ton cm−2 for 30 s. The specimens were isothermally sintered at 200 °C for 1 h and 550 °C for 2 h in pure argon atmosphere. The size of the testing specimens, containing 0–1 wt% of CNTs, are 20 mm in diameter and 10 mm in height.
2.3. Characterization
Microstructure of the Al/CNTs nanocomposites was characterized using field-emission scanning electron microscopy (FESEM-S4800 Hitachi). The composition and phase composition of Al/CNTs nanocomposite were analyzed by JEOL—JED 2300 energy dispersive x-ray analyzer and Bruker—D8 x-ray diffractometer. Brinel hardness test was performed to evaluate the hardness of the Al/CNTs nanocomposites.
3. Results and discussion
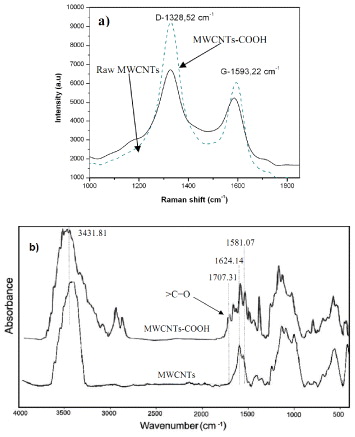
MWCNTs were chemically functionalized in HNO3:H2SO4: (1:3) at 60 °C for 6 h, so that the CNTs were well dispersed in ethanol solution. The existence of carboxyl (COOH) functional groups bonded to the ends and sidewalls, was demonstrated by Raman and Fourier transform infrared (FTIR) spectra as shown in figure 1.
Figure 1. (a) The Raman spectra and (b) FTIR spectra for the raw and carboxylically functionalized MWCNTs.
Download figure:
Standard image High-resolution imageFrom figure 1(a) it was clearly seen that the two bands around 1593 and 1328 cm−1 in the spectra were assigned to the tangential mode (G-band) and the disorder mode (D-band), respectively. The D-band intensity was increased in the modified MWCNTs compared to raw MWCNTs. The peak intensity ratio (ID/IG = 1.60) at D-band and G-band for the functionalized MWCNTs exceeded those of raw MWCNTs (ID/IG = 1.27). This result indicates that some of the sp2 carbon atoms ( ) were converted to sp3 carbon atoms (C–C) at the surface of the MWCNTs after the acid treatment in HNO3/H2SO4. The existence of carboxyl (COOH) functional groups bonded to the ends and sidewalls was demonstrated by FTIR spectra and is shown in figure 1(b). It shows an important peak after MWCNTs were treated by a mixture of H2SO4 and HNO3. The vibration of O–H bonding in carboxyl group is shown by peak 3431.81 cm−1. It expanded more than that of O–H bonding of H2O. Peak 1707.31 cm−1 shows the existence of vibration of
) were converted to sp3 carbon atoms (C–C) at the surface of the MWCNTs after the acid treatment in HNO3/H2SO4. The existence of carboxyl (COOH) functional groups bonded to the ends and sidewalls was demonstrated by FTIR spectra and is shown in figure 1(b). It shows an important peak after MWCNTs were treated by a mixture of H2SO4 and HNO3. The vibration of O–H bonding in carboxyl group is shown by peak 3431.81 cm−1. It expanded more than that of O–H bonding of H2O. Peak 1707.31 cm−1 shows the existence of vibration of  bonding in carboxyl group. This exhibits the importance of proving the existence of carboxyl (COOH) functional groups appearing due to the oxidation resulting from nitric and sulfuric acids. It clearly shows that the kinds of acids functionalized the surface of MWCNTs.
bonding in carboxyl group. This exhibits the importance of proving the existence of carboxyl (COOH) functional groups appearing due to the oxidation resulting from nitric and sulfuric acids. It clearly shows that the kinds of acids functionalized the surface of MWCNTs.
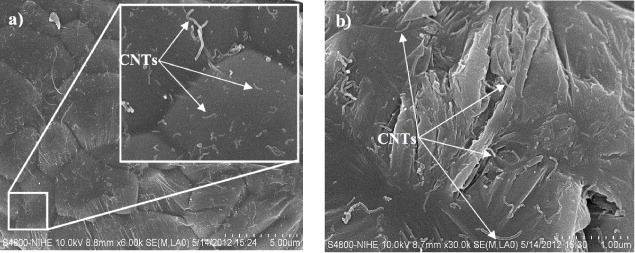
The Al powder modified by PVA was mixed into the CNTs suspension. By evaporating the ethanol and PVA component at 400 °C in argon atmosphere for 2 h, the obtained Al/CNTs nanocomposite powder shows homogeneously implanted CNTs on the surface of individual Al particle, as shown in figure 2(a). From this figure, the distribution of reinforcement of CNTs in the Al matrix can be observed. The Al/CNT nanocomposite powder was consolidated by sintering process to fabricate Al/CNTs nanocomposites. The surface morphology of Al/CNTs nanocomposites with 0.4 wt% of CNTs is shown in figure 2(b). It shows homogeneous distribution of carbon nanotubes within the Al matrix.
Figure 2. (a) SEM images of Al/CNTs nanocomposite powder, in which the CNTs are homogeneously implanted on the surface of individual Al particle and (b) surface morphology of Al/CNTs nanocomposites with 0.4 wt% of CNTs.
Download figure:
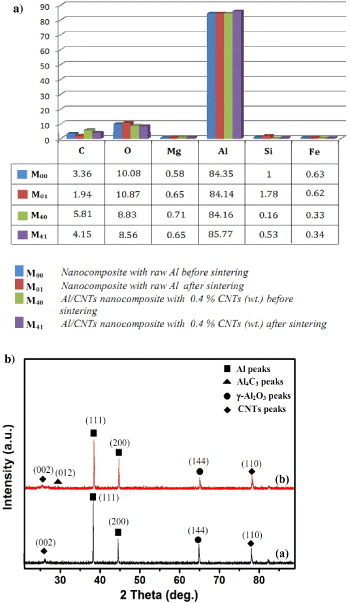
Standard image High-resolution imageEDX analysis results show that there exist not only two main compositions Al and C used for preparing Al/CNTs nanocomposite, but also some other elements like Fe, Si, Mg and O. Because the purity of Al powder only reaches to 99.5%, so these elements may be impurity elements in the preparation of Al powder process. Figure 3(a) shows the weight per cent of compositions graph of Al/CNTs nanocomposites obtained by EDX analysis. With Al/CNTs nanocomposite containing 0.4 wt% CNTs, the percentage of C element decreases after sintering and is still larger than that of nanocomposite without CNTs added.
Figure 3. (a) The weight percentages of compositions in Al/CNTs nanocomposite determined by EDX analysis. (b) XRD patterns of Al/CNTs nanocomposites with 0.4 wt% CNT added, (a)-line: before sintering and (b)-line: after sintering.
Download figure:
Standard image High-resolution imageXRD patterns of Al/CNTs nanocomposites with 0.4 wt% CNT added, (a)-line: before sintering and (b)-line: after sintering, are shown in figure 3(b). These results show the existence of the phase compositions of Al/CNTs nanocomposite such as Al, Al2O3, Al4C3 and CNTs. XRD study indicated that the CNTs peak at 2θ = 26° corresponds to the graphite (002) plane and the small intensity peak corresponds to (100) plane at 2θ = 42.4° and (110) planes at 2θ = 77.7° [27]. It is important to notice the unexpected presence of aluminum carbide (Al4C3) in the Al/CNTs nanocomposites after sintering. The aluminum carbide can be observed in the base line of XRD spectra at the small peak 2θ = 31.704° corresponding to (012) plane. This carbide was found in all MWCNTs concentrations. Previously, it has been reported that Al4C3 usually grows on the prismatic planes of the carbon fiber [19] and because of the natural positioning of C atoms on the CNTs shells, aluminum atoms feel in contact with a graphite basal plane, thus Al4C3 formation was not expected in Al/CNTs nanocomposites. Besides, the presence of Al phase was indicated at 2θ = 38.473° and 44.74° corresponding to (111) and (200) planes, respectively. In addition, the existence of γ-Al2O3 phase was shown at peak 2θ = 64.94° corresponding to (144) plane.
The fabricated Al/CNTs nanocomposite powders are consolidated into bulk Al/CNTs composite with full densification by compacting and sintering process. During sintering, the necks at the contact areas between the powder particles grew. As a consequence, a clear evolution of the pore morphology could be observed. The different shrinkage can then be attributed to the effect of the alloying elements on one or more of the following factors: the diffusion coefficients, the vapor pressure, the thermal expansion coefficients of the microstructural constituents and the volume change of the phase transformations upon cooling. During sintering, one of the punch surfaces was placed on such a plate, and the related frictional forces slightly opposed shrinkage. On the opposite surface, the shrinkage was free, such that some inclination occurred between the die surfaces. Table 1 shows the variation of density and relative density of the Al/CNTs nanocomposites with the mass fraction of CNTs. The nanocomposites with a high mass fraction of CNTs exhibited high porosity by hollow structure of CNTs. Therefore, the density of the Al/CNTs nanocomposites will be decreased.
Table 1. The dependence of theoretical, measured, relative density and hardness of Al/CNTs nanocomposite on the mass fraction of CNTs.
| CNTs content (wt%) | Theoretical density (g cm−3) | Measured density (g cm−3) | Relative density (%) | Hardness (HB) |
|---|---|---|---|---|
| 0 | 2.7 | 2.539 | 94 | 19.6 |
| 0.1 | 2.695 | 2.481 | 92.1 | 20 |
| 0.2 | 2.691 | 2.456 | 91.2 | 20.7 |
| 0.3 | 2.685 | 2.452 | 91.3 | 20.9 |
| 0.4 | 2.681 | 2.448 | 91.3 | 22.2 |
| 0.5 | 2.675 | 2.419 | 90.4 | 20.6 |
The hardness of the nanocomposites with different mass fractions of the CNTs is shown in table 1. The hardness was measured by Brinel hardness test. A considerable enhancement of the hardness is observed by addition of the CNTs in the Al matrix. The hardness increases almost linearly with increase of the CNT mass fraction up to 0.4%. At 0.4 wt% of CNTs added to nanocomposite, the hardness of the Al/CNTs nanocomposites reach to a value of 22.2HB, which is about 13% higher than that of the Al without CNTs. To explain the carrying capacity of the Al/CNTs nanocomposites, we assumed that Al matrix has the ability to transfer and distribute external force to the reinforcement material. The external force loaded on the Al matrix itself is reduced. Here, the reinforcement material is CNTs, which have high strength so that the Al/CNTs nanocomposites have mechanical durability greater than that of the raw Al matrix.
4. Conclusions
The successful functionalization of nanotubes with a mixture of acid solution proved by Raman and FTIR spectrum has opened up new applications in the composites field. A homogeneous distribution of MWCNTs in the Al matrix has been achieved by a novel processing approach based on the PDA method for preparing Al/CNTs nanocomposite. The dispersion of the CNTs in Al matrix is very important, even determining nanocomposite material properties such as enhancement of the mechanical behavior and wear resistance of Al/CNTs nanocomposites.
The density of nanocomposite decreases with increasing mass fraction of CNTs, and Al/CNTs nanocomposite with higher mass fractions of CNTs exhibited higher porosities by the hollow structure of CNTs. The hardness of the composite increases with increasing mass fraction of CNTs from 0 to 0.4% and decreases with more CNTs added. The introduction of CNTs in the Al matrix results in a decrease of the density of composite, whereas the hardness of composite increases by about 13% at the best reinforcement condition of 0.4 wt% CNTs.
Acknowledgments
The authors acknowledge financial support from the Research and Development of Technology program, Vietnam Academy of Science and Technology (VAST), project code: VAST.03.03/12-13 and VAST.HTQT.Nga.03/2012-2013. We would also like to thank the National Basic Research Fund, NAFOSTED on the projects code: 103.99-2012.15 and 103.99-2012.35 for the supporting fund for this research. This research was also supported by the fund of Young Scientist program of Institute of Materials Science, VAST.