Abstract
Polyaniline–mutilwalled carbon nanotube (PANi–MWCNT) nanocomposites were electropolymerized in the presence of sodium dodecyl sulfate (SDS) onto interdigitated platinum-film planar microelectrodes (IDμE). The MWCNTs were first dispersed in SDS solution then mixed with aniline and H2SO4. This mixture was used to electro-synthesize PANi–MWCNT films with potentiostatic method at E = + 0.90 V (versus SCE). The PANi–MWCNT films were characterized by cyclic voltammetry (CV) and scanning electron microscopy (SEM). The results show that the PANi–MWCNT films have a high electroactivity, and a porous and branched structure that can increase the specific surface area for biosensing application. In this work the PANi–MWCNT films were applied for covalent immobilization of glucose oxidase (GOx) via glutaraldehyde agent. The GOx/PANi–MWCNT/IDμE was studied using cyclic voltammetric and chronoamperometric techniques. The effect of several interferences, such as ascorbic acid (AA), uric acid (UA), and acetaminophen (AAP) on the glucosensing at +0.6 V (versus SCE) is not significant. The time required to reach 95% of the maximum steady-state current was less than 5 s. A linear range of the calibration curve for the glucose concentration lies between 1 and 12 mM which is a suitable level in the human body.
Export citation and abstract BibTeX RIS

Content from this work may be used under the terms of the Creative Commons Attribution 3.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Polyaniline (PANi) is one of the most intensively investigated conducting polymers due to its easy synthesis, well-behaved electrochemistry, good environmental stability, high conductivity, and strong biomolecular interactions [1, 2]. However, PANi expresses its conducting state only when it is in acidic medium, pH < 3.0 (emeraldine salt form) [3]. This pH condition is unfavorable for a biological system because most bioassays must be performed in neutral or slightly acidic conditions [2, 4]. Recent experimental studies have shown that the incorporation of carbon nanotubes (CNTs) into PANi matrix may significantly promote their mechanical and electrical conductivity both in reduced and neutral states [5]. Furthermore, PANi–CNTs composite has also been found to improve electrocatalytic activation of the redox enzymes via enhancing electrochemical transduction of biochemical processes [6, 7].
However, a major drawback is that CNTs are difficult to process, and they are insoluble in most solvents, which has limited the distribution of CNTs in the PANi matrix. These difficulties can be overcome using various surfactants such as sodium dodecyl benzenesulfonate (SDBS), dodecyltrimethyl-ammonium bromide (DTAB), hexadecyltrimethylammonium bromide (CTAB), octyl phenol ethoxylate (Triton X-100) and sodium dodecyl sulfate (SDS) [8]. These surfactants have been used not only for the dispersion of CNTs but also as a dopant for the synthesis process. Anionic surfactant SDS is widely used for this purpose [9–11].
In this study we synthesized the polyaniline–mutilwalled carbon nanotubes (PANi–MWCNTs) nanocomposite in the presence of SDS [9, 12] on interdigitated platinum-film planar microelectrodes (IDμE) by potentiostatic technique. The electrochemical glucose sensor based on PANi–MWCNTs is also described.
2. Experimental
2.1. Chemicals and reagents
MWCNTs (with >95% purity) were purchased from Shenzhen Nanotech Port (China). Glucose oxidase (GOx) EC 1.1.3.4 (from Aspergillus niger), 25% glutaraldehyde and SDS were used as received (Sigma Aldrich). Aniline (Merck) was distilled under reduced pressure and stored below 0 °C before use. β-d-(+)-glucose, H2SO4, KCl, KH2PO4, K2HPO4 and other reagents were of analytical grade and used without further purification. The glucose stock solution was allowed to mutarotate for 24 h in order to reach the equilibrium of α- and β- anomers at room temperature prior to use. Phosphate buffered saline (PBS, 20 mM KH2PO4 + 20 mM K2HPO4 + 0.1 M KCl, pH 6.8) was used as supporting electrolytes. All of the solutions were prepared with doubly distilled water.
2.2. Apparatus
Electrochemical measurements were performed on an Autolab PGSTAT12 Electrochemical Analyser (EcoChemie, Netherlands) under the control of GPES version 4.9. The experiments were carried out using a conventional three-electrode system with interdigitated planar platinum-film microelectrodes (IDμE) as the working electrode, a Pt wire as the auxiliary electrode, and a saturated calomel electrode (SCE) as the reference electrode. Field-emission scanning electron microscope (FE-SEM) images of the films were recorded with Hitachi S-4800 (Japan).
2.3. Fabrication of interdigitated platinum-film planar microelectrodes
The interdigitated platinum-film planar microelectrodes (IDμE) were fabricated on a silicon wafer using a photolithographic metal lift-off process. Two pairs of the interdigitated electrodes were prepared by sputtering 50 nm Cr and 200 nm Pt on a layer of silicon dioxide (SiO2) with a thickness of around 100 nm by means of dry thermal oxidation on top of the wafer. The microelectrode digit width was of 50 μm and the gap between them was 20 μm, and their length was around 1 mm (figure 1).
Figure 1. Planar IDμE device. (1) Insulating substrate, (2) microelectrode digit bars, (3) conductive lines and (4) contact pads.
Download figure:
Standard image High-resolution image2.4. Electropolymerization of PANi–MWCNT films
Two milligrams of MWCNTs were dispersed in 0.1 mM of SDS aqueous solution and sonicated for 1 h [8,13]. Then aniline monomer solution (0.01 M of aniline in 0.1 M of H2SO4) was dissolved in SDS and MWCNTs solution under ultrasonic stirring for 15 min at room temperature. The PANi–MWCNTs modified IDμE was fabricated by the potentiostatic method at a constant potential of +0.9 V versus SCE. After polymerization the electrode was rinsed with distilled water and placed into a PBS solution. For comparison, a PANi/IDμE was prepared under the same conditions.
2.5. Immobilization of glucose oxidase
Fresh solutions of glucose oxidase (GOx) were prepared in PBS and stored at 4 °C. The immobilization of GOx on the surface of the PANi–MWCNTs film was achieved by cross-linking through the glutaraldehyde agent. Firstly, the PANi–MWCNTs electrodes were placed in saturated glutaraldehyde vapor for 30 min then dried in air for 15 min at room temperature. Next, 8 μl of the GOx solution (2 U μl−1) was spread on the PANi–MWCNTs surface using a drop method. The product, the formed GOx/PANi–MWCNT/Pt electrode, was rinsed thoroughly with PBS solution and stored at 4 °C when not in use.
3. Results and discussion
3.1. Voltammetric behavior of the PANi–MWCNTs electrode
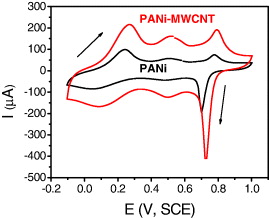
Cyclic voltammetry (CV) was used to characterize the redox properties of PANi–MWCNTs modified IDμE. The cyclic voltammograms obtained for both PANi and PANi–MWCNTs composite electrodes at a scan rate of 50 mV s−1 in the potential range of −0.20 to +1.0 V are shown in figure 2.
Figure 2. Cyclic voltammograms of PANi and PANi–MWCNTs electrodes in 1 M HCl at a scan rate of 50 mV s−1.
Download figure:
Standard image High-resolution imageThe current density of PANi–MWCNTs electrode is larger than that of the PANi electrode. Three identifiable anodic peaks with cathodic counterparts are observed for the PANi–MWCNTs electrode but not clearly for the PANi electrode. Here MWCNTs played an important role in increasing electroactivity and/or its specific surface area of the electrode. It significantly improves the overall biosensor performance.
3.2. Morphology
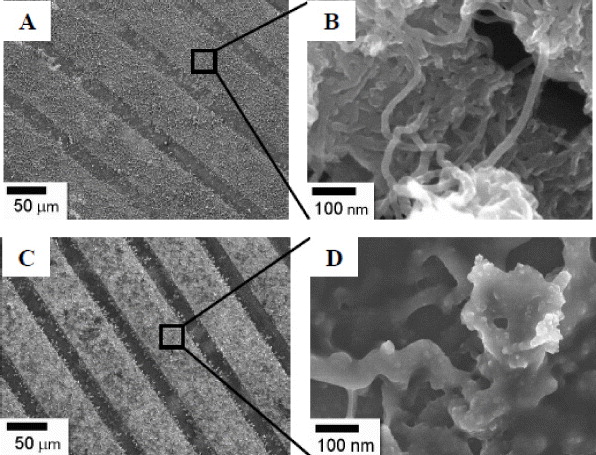
Figures 3(A) and (C) show the low magnification scanning electron micrographs obtained for PANi–MWCNTs and GOx/PANi–MWCNTs films on IDμE, respectively. The SEM image of PANi–MWCNTs shows spongy and porous morphology of polyaniline. The high magnification SEM of the PANi–MWCNT sample (figure 3(B)) indicates that carbon nanotubes dispersed in the polymer matrix and formed the homogeneous nanocomposites. It can be helpful in the entrapment of enzyme molecules. When GOx was added and allowed to covalently attach to PANi–MWCNT surface, the films became thicker and constituted a gel configuration (figure 3(D)). These small globules indicate the presence of enzyme (GOx) onto PANi–MWCNT surface.
Figure 3. FE-SEM images of the PANi–MWCNTs (A) and GOx/PANi–MWCNTs (C). High magnification SEM image (B and D), respectively.
Download figure:
Standard image High-resolution image3.3. Cyclic voltammograms of glucose on GOx/PANi–MWCNT/IDμE
Figure 4 shows the cyclic voltammograms (CV) of GOx/PANi–MWCNT/IDμE in the absence (curve a) and in the presence (curves b and c) of glucose in the PBS solution. The range of potential was from 0.0 to +0.8 V at a scan rate of 50 mV s−1.
Figure 4. Cyclic voltammograms responses at GOx/PANi–MWCNT/IDμE in PBS solution. (a) In the absence of glucose. Glucose added: (b) 1 mM and (c) 2.0 mM.
Download figure:
Standard image High-resolution imageThe CV curves of the electrodes corresponding to the addition of different glucose concentrations show an increase in anodic current when the potential varied from +0.2 to +0.8 V in comparison to that without glucose addition. It is worth noting that there are no changes in shape and position of the cyclic voltammograms when glucose concentrations were added. Here GOx has been successfully immobilized onto the PANi–MWCNT surface. It is also suggests that the range of potential response was due to hydrogen peroxide generation during the enzymatic reaction [14].
3.4. Selection of the applied potential
As can be seen in figure 4, the large response current was obtained within the range of +0.2 to +0.8 V. However, at high potential there may be interferences from other oxidable species such as ascorbic acid (AA), uric acid (UA), and acetaminophen (AAP). They commonly present in physiological samples of glucose and cause problems in the accuracy of the glucose determination. In this study the effect of the applied potential on amperometric response for glucose sensor was investigated.
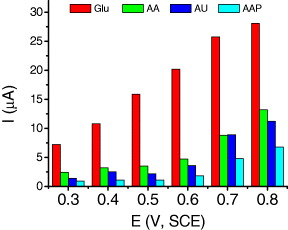
The amperometric responses of AA, UA, AAP, and glucose (1 mM) in the potential range of +0.2 to +0.8 V are shown in figure 5. Commonly, the susceptibility to interference species is positively correlated to the potential of the sensor. As a sequence, the lower potential results in the lower susceptibility and the increase of the sensor selectivity. When the applied potential is higher, the response current increases, however, it is strongly influenced by the interferences. When the applied potential increased from +0.6 to +0.7 V, the sensor currents of AA, UA, and AAP increased from 4.7 to 8.8, 3.6 to 8.89, and 1.9 to 4.8 μA, respectively. The errors in determination of 1 mM glucose at a potential of +0.6 V in solution caused by 1 mM AA, 1 mM UA, and 1 mM AAP are 22.38, 17.14 and 8.81%, respectively. Compared to the normal physiological levels that have been detected from blood samples, which are 0.13 mM for AA, 0.3 mM UA, and 0.125 mM for AAP [15], the biosensor exhibits essentially no response to such interfering species. Thus, the value of +0.6 V was chosen as applied potential of the GOx/PANi–MWCNT sensor.
Figure 5. Amperometric responses of AA, UA, AAP, and glucose (1 mM) in the range of 0.3– 0.8 V (versus SCE).
Download figure:
Standard image High-resolution image3.5. Amperometric determination of glucose at GOx/PANi–MWCNT/IDμE
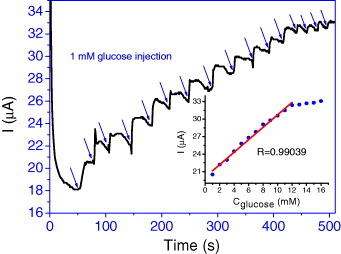
Figure 6 shows amperometric response at the GOx/PANi–MWCNT/IDμE in PBS at +0.6 V (versus SCE) for the successive addition 1 mM of glucose.
Figure 6. Amperometric response of GOx/PANi–MWCNT/IDμE upon increasing the glucose concentration in steps of 1 mM at +0.6 V (versus SCE) in PBS. Inset shows the calibration plot.
Download figure:
Standard image High-resolution imageThe bioelectrode exhibits a rapid and sensitive current response to each injection of glucose concentration. The time required to reach 95% of the maximum steady-state current was less than 5 s. The plot of current versus glucose concentration (inset, figure 3(C)) depicted a linear relationship over the range of glucose (1–12 mM) until saturation current at a high glucose-concentration (>12 mM). The linear regression equation was I (μA) = 1.07 × Cglucose (mM) + 20.06 with the correlation coefficient of 0.99039. This linear range is clinically sufficient for monitoring of the glucose levels in normal human blood, 4.4 − 6.6 mM [16].
4. Conclusion
PANi–MWCNTs nanocomposites were successfully synthesized in the presence of SDS. SDS acts as an agent not only to improve the solubility of aniline monomer but also to gain the well-dispersion of MWCNTs, and as counter ions for PANi. FE-SEM images confirm that the uniform coating of PANi–MWCNTs has been deposited onto IDμE surface. The porous property of PANi–MWCNTs was used as an immobilization matrix and a transducer for glucose biosensing. It was found that the resulting glucose biosensor exhibited a fast response (<5 s) in a wide linear range. These features provide scope for utilizing the methodology proposed in the present study to immobilize other biomolecules in the process of fabricating novel biosensors.
Acknowledgment
The authors are grateful for the financial support for this work by Hanoi University of Science and Technology (Code T2012–9)