Abstract
Nanostructured Ce0.5Zr0.5O2 samples were synthesized by the combustion of the gel obtained from a mixture of polyvinyl alcohol (PVA) and metal nitrates at a temperature as low as 600 °C. The prepared samples were investigated by x-ray diffraction (XRD) and field emission scanning electron microscopy (FE-SEM). Their specific surface areas were determined from N2 adsorption measurement at 77 K by Brunauer–Emmet–Teller (BET) method and their CO catalytic oxidation activities were investigated using a Landcom II instrument. The XRD and FE-SEM results revealed that Ce0.5Zr0.5O2 nanocrystallines began to grow isotropically at 600 °C with the nanostructure found in all prepared samples. Further thermal treatment at 600 °C for 2 h yields the single crystalline phase Ce0.5Zr0.5O2 nanostructured samples with average crystalline size < 50 nm and specific surface area of about 73 m2 g−1. Besides, the effects of a number of factors such as calcination temperature and Ce/Zr molar ratio on the formation of prepared samples as well as the oxidative conversion of carbon monoxide over nanostructured Ce0.5Zr0.5O2 catalyst were also studied.
Export citation and abstract BibTeX RIS

Content from this work may be used under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Nanostructured mixed oxides have been studied for applications in solid-oxide fuel cell, high temperature-furnace electrode, and as catalysts for the treatment of automobile exhausts [1, 2]. Cerium oxide and cerium-based mixed oxides play important roles in automotive three-way catalysts and oxidation catalysts because of their oxygen storage capacity. Nowadays, many studies on preparation and application of mixed CeO 2–ZrO 2 oxides have been reported [3–5]. For the preparation of a high performance heterogeneous catalysis, a synthesis route for these nanostructured mixed oxides that could be carried out at low temperatures and provide preparative materials with small grain size and a large specific surface, is crucially required. For these requirements, the conventional solid state reaction route seems to be unsuitable due to the fact that its reaction products can be normally formed only at high temperatures and exhibit small specific surface area [6, 7]. Among alternative synthesis routes of these nanostructured mixed oxides, the combustion synthesis method is a powerful preparative technique that enables obtaining nanostructured powder samples with uniform, fine grain size and large specific surface area at low temperature [4, 8–12].
This paper presents the results of synthesis and investigations on CO catalytic oxidation activity of Ce 0.5 Zr 0.5 O 2 prepared by combustion method using polyvinyl alcohol (PVA).
2. Experimental
2.1. Catalyst preparation
The nitrate salts of cerium and zirconium ions with a Ce/Zr molar ratio of 1:1 were mixed with appropriate amounts of the PVA solution (with mixed metal/PVA molar ratio=1:3) and the mixture was preheated at 80 °C to remove excess amount of water. The resulting viscous gel was placed in a furnace kept inside a fume hood and maintained at 600 °C for 2 h.
2.2. Catalyst characterization
Thermogravimetric (TGA) and differential thermal analysis (DTA) of the gel precursor and PVA were carried out on a Shimadzu TGA-50 and DTA-50 thermal analyser with a heating rate of 10 °C min −1 in air at temperature from room temperature to 700 °C. The crystalline phase analyse of the catalysts were carried out on a Siemens D 5000 diffractometer (Germany) with CuKα radiation at a step size of 0.03° in the 2θ range of 10–70°.
The morphology of the obtained samples was investigated by using a Hitachi S-4800 field emission scanning electron microscope (FE-SEM). Furthermore, the chemical composition of the preparation mixed oxides was analysed by using an energy dispersive x-ray analyser (EDX) attached to a JEOL-2300 scanning electron microscope.
The BET specific surface area of Ce 0.5 Zr 0.5 O 2 powder was determined by nitrogen adsorption at 77 K using an ASAP 2010 instrument (Micrometics, USA), operating in a single point mode.
2.3. Catalytic activity tests
The carbon monoxide oxidation conversion over nanostructured Ce 0.5 Zr 0.5 O 2 was carried out in a conventional flow reactor at ambient pressure and the reaction temperature ranged from room temperature up to 450 °C. For the oxidation of CO, 200 mg of the nanostructured Ce 0.5 Zr 0.5 O 2 was loaded inside a quartz tubular (inner diameter of 0.6 mm) fixed–bed reactor. 570 ppm CO was added to a gas mixture including 20 vol.% O 2 and 80 vol.% N 2 to serve as gas reactants. The total flow rate was 1000 cm 3 min −1, corresponding to a space velocity of 3000 cm 3 g −1 h −1. The CO concentration was analysed by a Landcom II instrument. The CO conversion efficiency was calculated as follows:
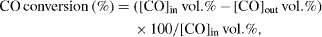
where [CO] out and [CO] in are the CO concentrations in the product (vol.%) and feed gas (vol.%), respectively.
3. Results and discussion
To obtain Ce 0.5 Zr 0.5 O 2 nanostructured powder, the precursor solution is consisted of desired metal ions dispersed in PVA polymeric reagent used as a matrix. For combustion preparative method, PVA is a suitable polymeric agent due to its ability to be decomposed at low temperature leaving behind a quite small amount of residual carbon, along with its high water solution. Moreover, it plays a dual role in combustion preparative method. Firstly, it enables the metal cations to be uniformly distributed throughout the viscous liquid during evaporation and as a result, it prevents the precipitation or separation of the metal cations from solution. Secondly, it serves as a fuel for auto combustion process.
3.1. Pyrolysis
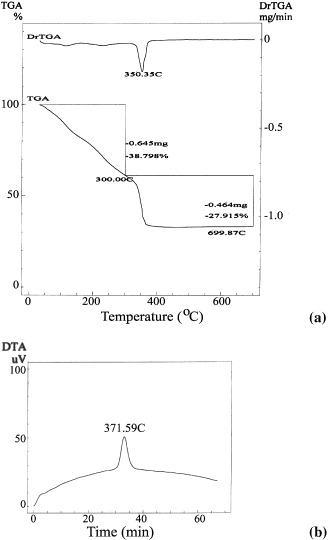
The pyrolysis of the gel precursor was monitored by thermal analyses as shown in figure 1. Two significant weight losses of 38.79 and 27.92% are found at 300 and 350 °C in the TGA curve, respectively. These weight losses might be associated with the removal of residual water and the decomposition of remaining PVA, respectively. This remaining amount of PVA is almost burned out at 372 °C with an exothermic signal in the DTA curve. No further changes in the TG curve are observed when heating the sample over 400 °C. This implies that the consumed heat of the samples provides the formation and growth of the mixed oxide only.
Figure 1 (a) TGA and (b) DTA curves of the as-prepared Ce 0.5 Zr 0.5 O 2 gel.
3.2. Effect of calcination temperature on nanostructured Ce 0.5 Zr 0.5 O 2 phase
Gels with mixed metal/PVA molar ratio=1:3 were prepared at 80 °C, pH=1, and subsequently calcined at various temperatures, namely, 450, 550, 600 and 650 °C.
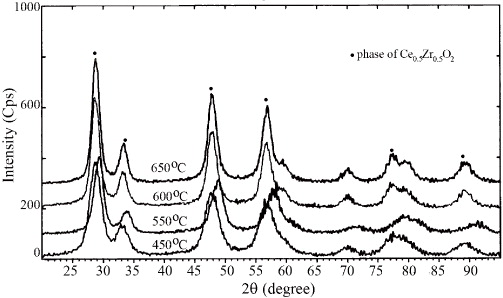
From XRD patterns of samples calcined at different temperature, it is obvious that Ce 0.5 Zr 0.5 O 2 crystallites started to grow isotropically for calcined temperature value of 600 °C due to the appearance of all available diffraction peaks coinciding with those in the standard pattern of Ce 0.5 Zr 0.5 O 2 single phase (figure 2). Hence, the calcined temperature of 600 °C was chosen to prepare desired samples (figure 3).
Figure 2 XRD patterns of the Ce 0.5 Zr 0.5 O 2 powder samples prepared by heating the precursor at different calcination temperatures for 2 h.
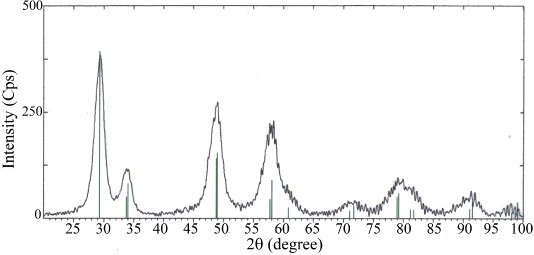
Figure 3 XRD diffractogram of the Ce 0.5 Zr 0.5 O 2 powder sample calcined at 600 °C for 2h.
3.3. Characterization of Ce 0.5 Zr 0.5 O 2 powder samples prepared by combustion method using PVA as a polymer matrix
The Ce 0.5 Zr 0.5 O 2 sample synthesized under selected conditions (gel formation temperature of 80 °C, pH=1, metal/PVA molar ratio=1:3 and calcined at 600 °C for 2 h) was examined by XRD, FE-SEM, EDX and BET measurements.
An SEM image of the Ce 0.5 Zr 0.5 O 2 powder sample showed that a homogenous nanostructure with a bees' nest formed and average crystalline grain size < 50 nm was obtained (figure 4). The three-dimensional distribution of pores is homogenous throughout the sample. With this nanostructure, the Ce 0.5 Zr 0.5 O 2 samples exhibit a specific surface area of 73 m 2 g −1.
Figure 4 (a) FESEM image and (b) EDX analysis result of the Ce 0.5 Zr 0.5 O 2 powder sample calcined at 600 °C.
EDX analysis results revealed that the purity of the prepared Ce 0.5 Zr 0.5 O 2 sample is at atomic level and its chemical composition was approaching the theoretical value (table 1).
Table 1. Chemical composition of the Ce 0.5 Zr 0.5 O 2 mixed oxide
| O | 21.69 | 20.77 |
| Zr | 30.85 | 30.92 |
| Ce | 47.46 | 48.21 |
3.4. Results of CO catalytic oxidation activity tests
3.4.1. Effect of Ce/Zr molar ratio on CO conversion
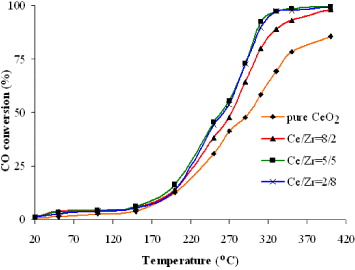
In order to explain why Ce 0.5 Zr 0.5 O 2 was selected as a catalyst for CO conversion among Ce x Zr 1−x O 2 mixed oxides, a series of Ce x Zr 1−x O 2 mixed oxides with different Ce/Zr molar ratios was prepared by combustion method and was subjected to CO catalytic oxidation activity tests. As shown in figure 5, a steep slope was observed in CO conversion curve for all studied samples. This could be explained by the fact that both Ce 3+ and Ce 4+ species coexisted in the above samples and Ce 4+ cations, with their high oxidation state, served as the catalytic active sites. As a result, CO conversion efficiency as high as 99% was achieved for Ce x Zr 1−x O 2 catalysts with Ce/Zr molar ratio ≤ 1. The results indicated that the catalytic activity of Zr-doped CeO 2 catalysts for the oxidation of CO is higher than that of pure CeO 2. Among all studied Zr-doped CeO 2 catalysts for CO conversion to CO 2, Ce 0.5 Zr 0.5 O 2 is the most active one.
Figure 5 Effect of the Ce/Zr molar ratio on CO conversion.
This result is similar to that of previous works reporting catalytic activity of Ce x Zr 1–x O 2 prepared by other synthesis routes [3, 5].
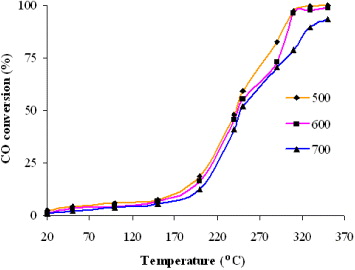
3.4.2. Effect of calcination temperature on CO conversion
Figure 6 shows the CO catalytic activities over the Ce 0.5 Zr 0.5 O 2 as a function of reaction temperature.
Figure 6 Effect of the calcination temperature on CO conversion.
From figure 6, it is found that the catalytic activity of studied Ce 0.5 Zr 0.5 O 2 samples decreased with the increase of the calcination temperature. This result agrees well with previous works [7]. Thus, the calcinations temperature is a key factor dominating the catalytic activity of the Ce 0.5 Zr 0.5 O 2 mixed oxide catalyst.
4. Conclusions
Nanostructured powder of Ce 0.5 Zr 0.5 O 2 was synthesized by combustion method at 600 °C for 2h using PVA as polymeric agent. With selected synthesis conditions (gel formation at 80 °C with pH of solution=1; metal/PVA molar ratio of 1:3), single crystalline phase of Ce 0.5 Zr 0.5 O 2 nanostructured powder was obtained with average crystalline grain size < 50 nm and specific surface area of about 73 m 2 g −1. Results of CO catalytic oxidation activity of the prepared samples showed that CO conversion efficiency strongly depends on calcination temperature, Ce/Zr molar ratio. The catalytic activity of these mixed oxides decreases with calcination temperature in the range of 500–700 °C.