Abstract
We have synthesized KxVy[Cr(CN)6]z. nH2O molecule-based magnet nanoparticles belonging to the Prussian blue (PB) family of compounds. The synthesized samples were characterized by infrared spectroscopy (IR), Raman spectroscopy, UV–Vis spectroscopy, differential thermal analysis (DTA) and thermogravimetric analysis (TGA). The crystal structure was refined from the x-ray powder diffraction profile by the Rietveld method. The samples are cubic, Fm3m space group with lattice parameter a=1.045 nm. The magnetic properties are determined from thermal variation of the magnetization and hysteresis loop. The most interesting result is the successful preparation of KxVy[Cr(CN)6]z. nH2O crystal Prussian blue nanomaterial which had Curie temperature (Tc) approaching room temperature.
Export citation and abstract BibTeX RIS

Content from this work may be used under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
The synthesis of materials with new and predictable properties is a challenge for chemists and physicists. A few years ago, inorganic molecular chemists were engaged in the synthesis of high-temperature molecule-based magnets, and this area remains very active. Presently, two families of molecule-based materials behave as room-temperature magnets. The first family can be formulated as V[TCNE]x·yCH 2 Cl 2 and was obtained by Manriquez et al [1]. However, the compound decomposes before reaching TC , and up to now it has not been fully characterized [2]. The second family of materials that behave as room-temperature magnets, V[Cr(CN)6]x·nH 2 O, was obtained in 1995 by Ferlay et al [3]. This amorphous Prussian blue(PB) analogue has a Curie temperature of 315 K and a low magnetization at saturation (0.15 μ B ). It is an air-sensitive compound that contains V II and V III cations. Other members of the PB family with vanadium and chromium cations have since been obtained by other authors and these have higher Curie temperatures and saturation magnetizations in line with their stoichiometry [2–5]. Two significant improvements of the synthetic procedure have led to better organized samples. Thus, a sol-gel approach and the use of potassium countercations allowed Girolami and Holmes to obtain a crystalline analogue with which a T C value of 376 K was reached, the highest in the series [6]. On the other hand, the use of catalytic amounts of V III during the synthesis allowed Garde et al to obtain stoichiometric Cr III V II compounds presenting the expected magnetization at saturation [7]. In this paper we report on the synthesis, sample characterization and crystal structure of vanadium–chromium PB analogues. The x-ray diffraction (XRD) pattern analysis indicated that these compounds possessed a face-centered cubic (fcc) crystal structure, Fm3m space group with lattice parameter a=1.045 nm. The resultant products were characterized by infrared spectroscopy (IR), Raman spectroscopy and UV–Vis spectroscopy. The magnetic properties are determined from thermal variation of the magnetization and hysteresis loop. Results of the study of low temperature magnetic relaxation in the temperature range with strong irreversibility in zero-field-cooling (ZFC) and field-cooling (FC) magnetization are presented and discussed.
2. Experimental
The synthesis of two vanadium-chromium PB analogues has been carried out by a route similar to that described in [8] but with some modifications. K x V y [Cr(CN)6]z ⋅nH 2 O samples were obtained by mixing saturated aqueous solution K 3[Cr(CN)6] and aqueous solution VCl 2 under anaerobic conditions. A deep blue precipitate occurred immediately; it was left in its mother solution for one day under argon. The blue precipitate was then filtered and washed several times with distilled water. Finally, the sample was dried under a vacuum for 6 h, at 60 °C.
Structural characterization was analyzed by means of XRD using a D5005 diffractometer at room temperature with CuKα radiation (λ=1.54056 Å). The infrared spectra were recorded under argon on a Nicolet 6700 FTIR spectrophotometer, in the 4000–400 cm −1 range with KBr pellets containing 1% of the sample in mass. Raman measurements were performed in a back scattering geometry using a micro-Raman LABRAM-1B and 632.8 nm line of He–Ne ion laser. The magnetic properties of the samples were investigated by a Physical Property Measurement System (PPMS) 6000 (Quantum design).
3. Results and discussion
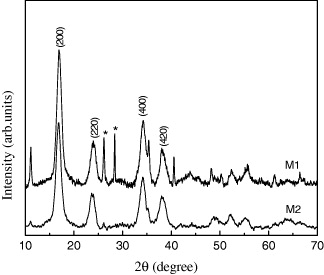
The powder XRD patterns of the samples are shown in figure 1. Compounds M1 and M2 are crystalline, and their diffraction patterns can be indexed to face-centered cubic (fcc) unit cells with a lattice constant of approximately 1.045 nm. Estimation of the mean size of the PB particles is performed from the width of the (200) Bragg reflection using the Debye–Scherrer equation. The average crystallite sizes are about 8 and 10 nm for M1, M2, respectively. Moreover, there are two peaks (symbol * in figure 1) for M1, which were introduced from the starting material and were not completely washed out by water or ethanol.
Figure 1 XRD of PB K x V y [Cr(CN)6]z ·nH 2 O.
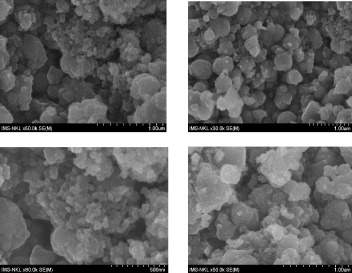
Figure 2 shows typical field emission scanning electron microscopy (FE-SEM) images of the obtained products. From these images we can clearly see that K x V y [Cr(CN)6]z ·nH 2 O particles are cubic in shape. The particle size of the sample is about 50 to 200 nm.
Figure 2 FE-SEM images of PB K x V y [Cr(CN)6]z ·nH 2 O.
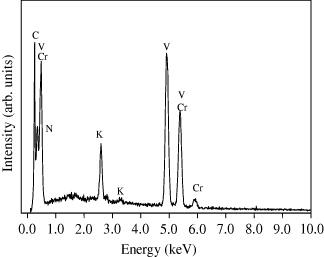
The composition of the sample is confirmed by energy-dispersive x-ray (EDX) analysis, which reveals the presence of C, N, O, V, Cr and K. Figure 3 shows the EDX spectrum of the sample. The EDX data of the sample are shown in table 1. The result proves that the V:Cr ratio is about 1.64.
Figure 3 EDX spectrum of the sample.
Table 1. EDX data of the sample.
| C K | 15.22 | 30.93 |
| N K | 21.89 | 38.16 |
| Al K | 0.08 | 0.07 |
| K K | 0.38 | 0.23 |
| V K | 35.97 | 17.24 |
| Cr K | 22.34 | 10.49 |
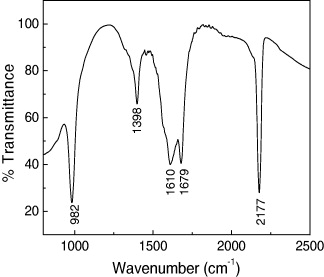
The formation of PB was confirmed by the Fourier transform infrared (FTIR) spectroscopy of the K x V y [Cr(CN)6]z .nH 2 O sample. Cyano complexes can be easily identified since they exhibit sharp ν(CN) at 2200–2000 cm −1 [2, 9]. Figure 4 shows the IR spectrum of the compound in the 900–2500 cm −1 range. In this part of the IR spectrum ν as (C≡N) asymmetric stretching bands appear, which are sensitive to the bonding mode of the cyanide and to the oxidation state of the metallic ions coordinated to the CN bridge. The ν(CN) of free CN - is 2080 cm −1 (aqueous solution). In coordination with a metal, the ν(CN) shift to higher frequencies [10, 11]. The absorption band at 2177 cm −1 shows common characteristics of PB and its analogues, corresponding to the stretching vibration of the CN group. It can be assigned to V II –N≡C–Cr III sequences, where the Cr III ion is bonded to the carbon and the V II ion is bonded to the nitrogen of the cyanide ligand [4]. There are two bands, one band at 1679 cm −1, corresponding to a δ(H–O–H) sequence, and another band at 1610 cm −1, assigned to a δ(O–H) sequence [11], indicating the presence of interstitial water in the samples. The band at 982 cm −1 is assigned to ν(V=O) stretching [4].
Figure 4 Infrared spectrum of the sample.
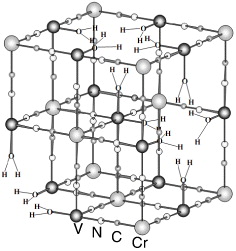
In the IR spectrum δ(H–O–H), δ(O–H) and ν(V=O) stretching bands appear. It has been reported that the PB analogues have Cr(CN)6 vacancies and the oxygen of H 2 O fills the empty nitrogen sites of the vacancy (figure 5). The mean number of Cr neighbors around V is less than six. The less vacancies there are, the higher T C is [2].
Figure 5 Certain Cr(CN)6 sites are vacant, and the bridging oxygen of H 2 O fills the empty nitrogen.
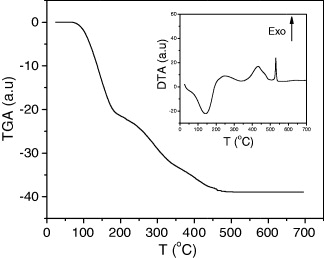
The results of TGA and DTA are shown in figure 6. TGA was carried out to determine the number of associated water molecules per formula unit. The TGA curve indicates that the samples lose water from room temperature up to about 180 °C. A plateau is observed between 180 and 240 °C. The total mass loss up to the midpoint of the plateau corresponds to the mass of all water molecules. At about 240 °C, a break corresponding to the decomposition of the compounds occurs. The DTA curve shows an endothermic process at about T=141 °C corresponding to water loss and an exothermic process at about T=530 °C corresponding to sample decomposition.
Figure 6 TGA and DTA curves of PB K x V y [Cr(CN)6]z ·nH 2 O.
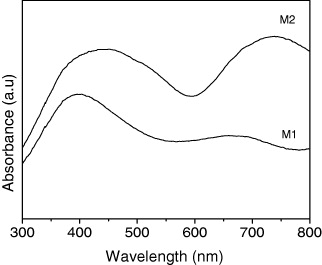
Figure 7 shows the UV–Vis absorption spectra of PB K x V y [Cr(CN)6]z ·nH 2 O particles. In the case of M1, the maximum absorbance appears at 403 nm and for M2 it appears at 452 nm. They are assigned to the ligand-to-metal charge-transfer band of the Cr II –C≡N–V III unit, which was produced by the reduction of some Cr III –C≡N–V II units. It corresponds to the mixed-valence charge-transfer absorbance of V and Cr [12].
Figure 7 UV–Vis spectra of the samples.
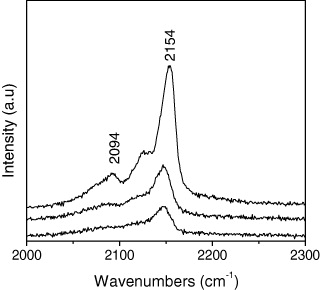
The M2 sample was also studied dynamically using Raman spectroscopy as shown in figure 8. There are two strong vibrational bands at 2094 and 2154 cm −1, respectively, which are most likely caused by the stretching vibration of the C≡N group of PB [9, 13]. The cyanide stretching modes are known to be sensitive to the oxidation state of the coordinating metals. Therefore, changes in the Raman spectra are due to oxidation state changes in V and Cr. Cyanide coordinated to Cr(II) has a single resolvable low-wavenumber peak at 2094 cm −1, which could be assigned to ν(Cr II –C≡N–V III ). Cyanide coordinated to Cr(III) has three high-wavenumber peaks at 2154 cm −1, which could be assigned to ν(Cr III –C≡N–V II ). This analysis indicates various valence states of both V and Cr exist, resulting in the effect on the exchange interaction, consequently affecting the transition temperature [14].
Figure 8 Raman spectra at three various points of the sample.
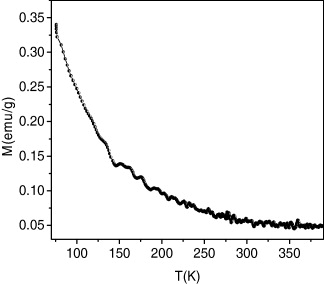
The thermal variation of the magnetization for the compound is shown in figure 9. The field-cooled magnetization versus T curve, in an applied field of 1000 Oe, presents a break at 276 K, confirming the presence of a magnetic order below this temperature. The highest Curie temperature, achieved in a chromium vanadium ferrimagnet, containing vanadium (II) and chromium (III), is about 315 K [3, 15]. A simple model, based on the magnetic orbital symmetry and energy, can rationalize this high value of T C . The molecular field theory developed by Neel for ferrimagnets with cubic structure is a valuable tool to analyze the relationship between the critical temperature, T C , the mean number of magnetic nearest neighbors, z, the exchange parameter between two metal ions (through the bridging ligand), J (H=−JS1 S2), and the metal ion spin values, S1 and S2 [2, 4, 16]. The exchange parameter J is related to the molecular mean field parameter W that expresses the interaction between the magnetic moments supported by two independent sublattices forming the 3D material:
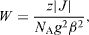
where N A is Avogadro's constant, β is the Bohr magneton, and g is the mean g factor. For PB analogues M'[M(CN)6]y, the critical temperature T C is given by
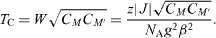
The Curie constants
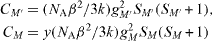
correspond to one M' and yM, respectively. The number of nearest neighbors of M' is z. Its mean value is equal to 6y in PB analogues. The expression is valid for ferro- or ferrimagnets when the interaction with the second nearest neighbors can be neglected [4]. This is a reasonable assumption for the compound, where the next neighbors are at 10.45 Å.
Figure 9 Thermal variation of the magnetization of the sample.
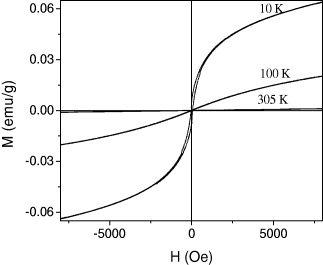
The hysteresis loops at different temperatures for M1 are given in figure 10. The results indicate that at 10 and 100 K, the compound is ferrimagnetic material. At 300 K, the compound is paramagnetic material. This analysis is a reasonable result of the thermal variation of the magnetization as shown in figure 9.
Figure 10 Hysteresis loops of the sample.
4. Conclusion
We have presented the structural and magnetic properties of vanadium–chromium PB analogue K x V y [Cr(CN)6]z . It is a member of hecxacyanometallate-based molecular magnetic nanomaterials. It crystallizes in the face-centered cubic structure with space group Fm3m and lattice parameter a=1.045 nm. The compound is a ferrimagnetic material with Curie temperature of about 276 K. The V:Cr ratio priori is the most important parameter for the magnetic properties of the compound. The less vacancies there are, the higher z and |J| are, and therefore T C reaches a higher temperature. This study has shown that a strong relationship exists between structure, disorder and magnetic behavior in Prussian blue analogues.
Acknowledgments
We would like to thank the Center for Nanoscience and Technology of Hanoi National University of Education and the Institute of Materials Science of Vietnam Academy of Science and Technology for support of this research.