Abstract
The dispersion of carbon nanotubes (CNTs) in liquid plays an important role in fundamental research and applied science. The most common technique applied to disperse CNTs is ultrasonication. The surfactants used for CNT dispersion are ethanol, sodium dodecyl benzenesulfonate (SDBS), dodecyltrimethylammonium bromide (DATB), sodium dodecyl sulfate (SDS) and sodium dodecylbenzene sulfonate (NaDDBS). This paper presents the dispersion of denatured CNTs by using a dimethylformamide (DMF) solution. The DMF is adsorbed on the surface of the nanotubes by a hydrophobic or π–π interaction. Ultrasonication helps DMF debundle the nanotubes by Coulombic or hydrophilic interaction, allowing the Van der Waals forces among the individual nanotubes to be overcome. UV–Vis spectra of dispersed CNTs in solution showed a maximum at 260 nm and decreased from UV to near IR. The vibration properties of the carbon samples were characterized with Raman spectroscopy, which illustrated the D and G bands of denatured CNTs at 1354 and 1581 cm−1, respectively, different from the values of 1352 cm−1 and 1580 cm−1, respectively, for undenatured CNTs. Finally, the interaction between surfactants and nanotubes was studied by Fourier transform infrared spectroscopy (FTIR).
Export citation and abstract BibTeX RIS

Content from this work may be used under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 3.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
Carbon nanotubes (CNTs) are 1-D nano-materials and interesting molecules, and were initially illustrated by Iijima [1]. They are structurally unique materials that exhibit excellent mechanical, electrical, thermal and optical properties [2], and are promising for a number of novel applications including chemical sensors, field emission devices, electrochemical devices, nanotube-based lamps [3], DNA sensors [4] and nanocomposits [5]. Certain applications require pure and well-isolated individual CNTs, therefore, a rather extensive effort has been devoted to achieving good dispersion of CNTs through chemical functionalization [6, 7] and physical interactions [8]. The former has been found to be effective but it deteriorates the intrinsic properties of the CNTs. Dispersing CNTs and obtaining their stable suspensions is an essential factor for their utilization in solution-phase separation methods [9], controlled deposition [10] and incorporation into composite materials [11]. The Van der Waals attraction forces between the nanotubes result in the formation of bundles, ropes and agglomerates. Thus, mechanical treatments as well as chemical functionalization (covalent and non-covalent bonding) have been proposed to improve the dispersion ability of the carbon nanotubes in both organic and highly polar solvents. The mechanical approaches, based primarily on high-power sonication, are capable of effectively segregating the CNTs. However, these methods can also cause a shortening of the nanotubes and decrease their aspect ratio. The covalent chemical modifications involve the attachment of various moieties that increase their solubility in the solvents. These approaches include various routes, e.g. controlled oxidation with strong acids [12] or organic peroxides [13]. The covalently attached moieties not only change the chemical properties but also alter the electrical properties of the CNTs [14]. Contrary to the covalent approach, non-covalent modification is more attractive because it relies on the adsorption of various molecules onto the CNTs' surface without disturbing their internal electronic structure. A wide variety of surfactants (anionic and non-ionic) have been studied for preparing stable dispersions of carbon nanotubes, e.g. sodium dodecyl sulfate (SDS) [10], sodium dodecyl benzenesulfate (SDBS), dodecyltrimethylammonium bromide (DATB) [15], sodium dodecylbenzene sulfonate (NaDDBS) [16], hexadecyltrimethylammonium bromide and DNA solution [17].
In this work, we report a facile method to disperse denatured CNTs in dimethylformamide (DMF) solution. The UV–Vis spectra were used to study the dispersion of the CNTs in the solution. The vibration properties of the carbon samples are characterized by Raman spectroscopy and the interaction between the surfactants and the CNTs is also studied by Fourier transform infrared (FTIR) spectroscopy.
2. Experimental
All the chemicals used were of analytical grade. The CNTs were produced by Shenzhen Nanotech Port Co. Ltd. (diameter <10 nm, length 5–15 μm, purity: ∼95%, amorphous carbon <3%), dimethylformamide (DMF) provided by Merck Chemical Co, nitric acid HNO 3 65% was purchased from Sigma.
CNTs can be synthesized by the thermal chemical vapor deposition method [18]. The experiments for the dispersion of the CNTs were performed in the following way. Firstly, 100 mg of CNTs were denatured in 50 ml concentrated nitric acid (15 M) and refluxed for 8 h in a silicone oil bath maintained at 110 °C to form carboxylic groups. Secondly, the denatured CNTs were rinsed with distilled H 2 O until the pH of the solution was neutral and then they were dried at 80 °C in a vacuum oven. Subsequently, 15 mg of the denatured CNTs were dispersed in 30 ml DMF solution in a flask. The ultrasonication processes were carried out at a power of 125 W for 120 min and, finally, the CNT solution was centrifuged at 2000 rpm for 5 min.
UV–Vis absorption spectra were recorded with a VARIAN UV–Vis spectrometer operating between 190 and 900 nm. Hitachi S4800 field emission scanning electron microscopy (FE-SEM) was used to characterize the morphology of both the denatured and pristine samples. FTIR spectra were measured on the Thermo Nicolet 6700 spectrometer. Raman spectra were recorded on a REFUSHAW Raman spectrometer operating at 633 nm laser light.
3. Results and discussion
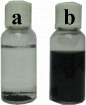
In order to determine the dispersibility of the denatured CNTs in DMF solution, we performed experiments with both pristine CNTs and denatured CNTs at the same conditions. Figure 1 shows the optical image of the pristine (a) and denatured (b) CNTs dispersed in the DMF solution for 120 min of ultrasonication at 125 W. We have found that the pristine CNTs dispersed in the DMF solution formed sediment at the bottom of the vessel after 2 months. In contrast, the denatured CNTs were completely dissolved and there was no sediment observed at the bottom of the jar. This can be explained by the presence of carboxyl groups, which are supposed to be directly attached to the wall of the CNTs and increase the dispersion of the CNTs in the solvent.
Figure 1 The optical image of the pristine (a) and denatured (b) CNTs dispersed in the DMF solution.
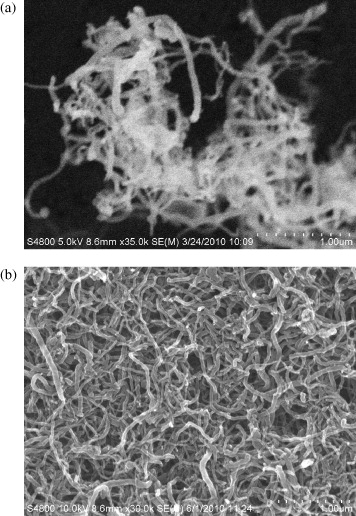
The FE-SEM images of the early CNTs and denatured CNTs are compared in figure 2. It is found that the early CNTs exist in the form of big bundle or a rope of CNTs (figure 2(a)), which are closely attached each to other when dispersing, due to the fact that the CNTs are very long and easy to entangle. Moreover, the Van der Waal forces between the tubes are the same as ones in the graphite structure. This causes a large absorption force between the fibers preventing the CNTs from dispersing. However, the inverse happens for the denatured CNTs: the tubes are dissociated from the bundles to form individual tubes as presented in figure 2(b). Thus, the denatured CNTs have a high degree and uniform dispersion in the DMF solution.
Figure 2 The FESEM image of pristine (a) and denatured (b) CNTs dispersed in DMF.
The dispersion of the CNTs in the solution can also be studied by UV–Vis spectroscopy. As mentioned in [10], the individual CNTs are active in the UV–Vis region but the bundled CNTs are less active at wavelengths from 190 to 900 nm. Thus, it is possible to establish a relationship between the dispersed CNTs and the intensity of the correlative absorption spectra. As illustrated in figure 3, the UV–Vis spectrum of the denatured CNTs in the DMF solution (0.5 mg ml −1, 120 min sonication at 125 W) shows a maximum at 260 nm and decreases from UV to near IR (figure 3(b)). At the same condition, the undenatured CNTs are low-dispersed because of the existing bundles, leading to a very low absorbance in their UV–Vis spectrum (figure 3(a)).
Figure 3 UV–Vis spectra of the pristine (a) and denatured (b) CNTs in DMF.
Raman spectroscopy [19] has been used to investigate the influence of the dispersion on the vibrational properties of the carbon samples. Figure 4 shows the Raman spectra of the pristine and denatured CNTs excited by the source with a wavelength of 633 nm, power of 4 mW and an operational bandwidth from 800 to 2000 nm. The Raman spectrum (a) shows characteristic bands, a D band at 1352 cm −1 and a G band at 1580 cm −1. The G band is associated with stretching vibrations of the carbon–carbon bonds in graphene layers. The D band is attributed to the presence of defects (sp 3 carbons, vacancies, foreign atoms, etc). The intensity of the G and D bands are a commonly accepted indicator of the sample crystallinity. Figure 4(b) shows the D band at 1354 cm −1 and the G band at 1581 cm -1 for the denatured CNTs. As one can see, the denatured CNTs exhibit a small increase of frequency but a clear decrease of the intensity of both the D and G bands, which could be caused by the lower CNT concentration in the solution. We note also that both the pristine and denatured samples show a low G/D intensity ratio, which means a low crystallinity of the MWCNTs samples. The denatured sample shows an even lower G/D ratio due to the defects created along the nanotube surfaces during the vigorous acid treatment.
Figure 4 Raman spectra of dispersed CNTs in DMF solution (a) before and (b) after denaturation. Excitation with wavelength of 633 nm, power of 4 mW.
Fourier transform infrared spectroscopy was used to study the interactions between the surfactants and the nanotubes. Figure 5 shows the FTIR spectra of the pristine and denatured CNTs in the range of 600–4000 cm −1. For the pristine tubes (figure 5(a)) there is a broad absorption band with a small peak at 3629 cm −1 due to the stretching vibration of O–H [10], while the peak at 1721 cm −1 is attributed to C=O bond stretching. The deformation band at 1123 cm −1 belongs to O–H bond, while other bands at 968, 867 and 659 cm -1 indicate the bending frequencies of the O–H bond. On the other hand, for the denatured CNTs (figure 5(b)), the characteristic peak of the hydroxyl group is shifted to 3636 cm −1. This band might have been due to both water and OH functional groups resulting from the chemical treatment during the purification and denaturation processes, respectively [20]. The peak at 1734 cm −1 is associated with the C=O stretching of the carboxylic acid (–COOH) group [21]. The peak at 1631 cm −1 is normally attributed to C=C stretching of the CNTs [22], but it may be associated with the N–H bond as well. The peaks at 1129 and 1010 cm −1 correspond to the C–O bonds. The peaks at 930, 862 and 657 cm −1 are attributed to the C–H bonds. These results indicate that denaturation of the carbon nanotubes had occurred, leading to the modification of the surface atoms as well as the nature of the functional groups.
Figure 5 FTIR spectra of the CNTs dispersed in DMF solution: (a) pristine CNTs and (b) denatured CNTs.
4. Conclusion
This paper presented a technique for the dispersion of CNTs in DMF solution. For the MWCNTs to be dispersed in the solution they were oxidized using strong nitric acid. The dispersion results were characterized by UV–Vis spectra which showed a clear increase in the absorption compared to pristine CNTs and with a maximum at 260 nm. Raman spectroscopy and Fourier transform infrared spectroscopy also showed that the denatured MWCNTs have a much better dispersibility in DMF solution than the pristine tubes. The method developed here can be useful for when CNTs are used for fabricating composite materials or when the applications require individual nanotubes.
Acknowledgment
The work was supported by the Ministry of Education and Training under research Project code B2010-01-405-TD.